Impact of high-density lipoprotein cholesterol level on early vascular healing following implantation of drug-eluting stent
-
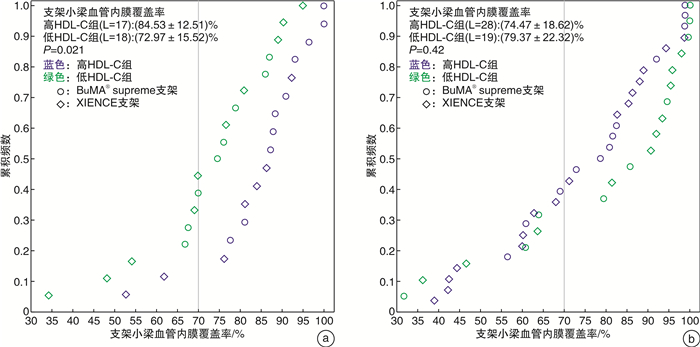
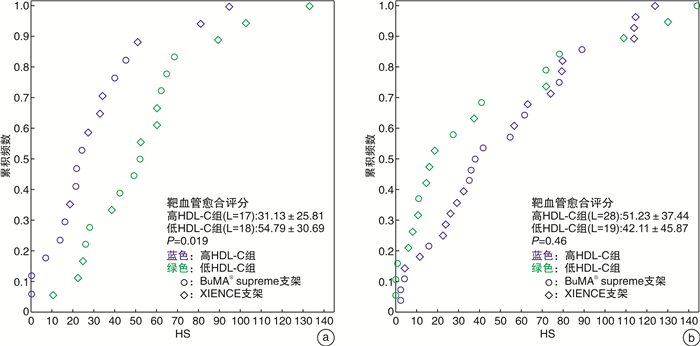
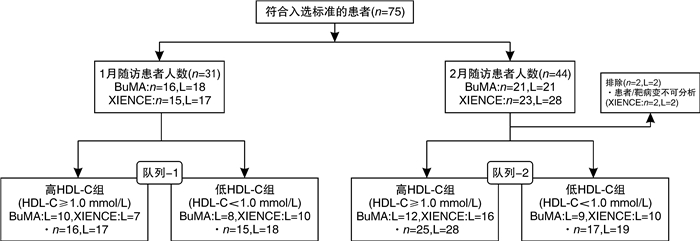
摘要: 目的 在动脉粥样硬化性心血管疾病(ASCVD)患者植入药物洗脱支架(DES)后第1和第2个月,使用光学相干断层扫描(OCT)研究基线高密度脂蛋白胆固醇(HDL-C)水平对早期血管愈合的影响。方法 此回顾性研究建立于PIONEER-Ⅱ OCT临床试验(ClinicalTrials.gov Identifier:NCT02747329)基础上。不论DES的品牌,根据基线HDL-C水平对每个队列中存在高出血风险的患者进行分组(高HDL-C水平≥1.0 mmol/L,低HDL-C水平 < 1.0 mmol/L)。队列1中的患者在经皮冠状动脉介入治疗(PCI)后1个月行OCT成像(高HDL-C组:16例患者,17个血管病变;低HDL-C组:15例患者,18个血管病变);队列2中的患者在PCI术后2个月行OCT成像(高HDL-C组:25例患者,28个血管病变;低HDL-C组:17例患者,19个血管病变)。结果 在第1个月随访时,高HDL-C组的支架小梁血管内膜覆盖率明显高于低HDL-C组(P=0.021),愈合评分显著优于低HDL-C组(P=0.019);在第2个月随访时,高HDL-C组与低HDL-C组之间支架小梁血管内膜覆盖率和愈合评分均差异无统计学意义(P=0.42、0.46)。结论 在DES植入后1个月,基线HDL-C水平≥1.0 mmol/L的患者比基线HDL-C水平 < 1.0 mmol/L的患者有更好的支架小梁血管内膜覆盖;但是在DES植入后第2个月的随访患者中,支架小梁血管内膜覆盖的差异不显著。这表明在DES植入后靶血管愈合的过程中,较高的HDL-C水平很可能促进了早期(术后1个月)新生内膜覆盖。
-
关键词:
- 高密度脂蛋白胆固醇 /
- 药物洗脱支架 /
- 稳定型心绞痛 /
- 支架小梁血管内膜覆盖 /
- 光学相干断层扫描
Abstract: Objective To investigate the impact of baseline high-density lipoprotein cholesterol(HDL-C) level on early vascular healing of drug-eluting stent(DES) in patients with arteriosclerotic cardiovascular disease(ASCVD), using optical coherence tomography(OCT) at the first and second month after stenting.Methods This retrospective study was based on the PIONEER-Ⅱ OCT trial(ClinicalTrials. gov Identifier: NCT02747329). Regardless of DES type, we grouped patients with high bleeding risk in each cohort based on baseline HDL-C levels(high level≥1.0 mmol/L; low level < 1.0 mmol/L). Patients in cohort-1 underwent OCT imaging 1 month after percutaneous coronary intervention(PCI)(high HDL-C group: 16 patients with 17 lesions; low HDL-C group: 15 patients with 18 lesions); Cohort-2 underwent OCT imaging 2 months after PCI(high HDL-C group: 25 patients with 28 lesions; low HDL-C group: 17 patients with 19 lesions).Results At the first month of follow-up, the strut coverage in the high HDL-C group was significantly higher than that in the low HDL-C group(P=0.021), and healing scores showed better healing than that in the low HDL-C group(P=0.019). At the second month of follow-up, the strut coverage and healing score were not significantly different between the high and low HDL-C groups(P=0.42, 0.46).Conclusion Patients with higher baseline HDL-C levels(≥1.0 mmol/L) had a greater strut coverage than patients with lower baseline HDL-C levels(< 1.0 mmol/L) 1 month following DES implantation, but the difference in strut coverage became less evident at the second month, which suggests that higher HDL-C enhances early neointimal coverage in the vascular healing process following DES implantation. -

-
表 1 入组患者的一般基线资料
Table 1. Baseline characteristics of enrolled patients
例(%), X±S 项目 队列-1 队列-2 高HDL-C组(16例) 低HDL-C组(15例) P 高HDL-C组(25例) 低HDL-C组(17例) P 男性 10(62.5) 9(60.0) 1.0 12(48.0) 12(70.6) 0.15 年龄/岁 63.75±9.68 61.27±8.30 0.45 62.88±7.60 64.82±12.67 0.58 糖尿病 7(43.8) 3(20.0) 0.25 5(20.0) 6(35.3) 0.27 高血压 11(68.8) 10(66.7) 1.0 19(76.0) 13(76.5) 1.0 肾功能衰竭 0(0.0) 0(0.0) 0(0.0) 0(0.0) 心房颤动 0(0.0) 0(0.0) 0(0.0) 2(11.8) 0.16 有出血史 3(18.8) 2(13.3) 1.0 3(12.0) 0(0.0) 0.26 有脑血管疾病史 3(18.8) 3(20.0) 1 8(32.0) 2(11.8) 0.25 有心肌梗死病史 2(12.5) 0(0.0) 0.48 0(0.0) 0(0.0) 既往接受过PCI术 0(0.0) 1(6.7) 0.48 0(0.0) 0(0.0) 既往接受过CABG术 0(0.0) 0(0.0) 0(0.0) 0(0.0) 临床表现 无明显心绞痛 3(18.8) 1(6.7) 0.60 2(8.0) 2(11.8) 1.0 稳定型心绞痛 3(18.8) 2(13.3) 1.0 5(20.0) 3(17.6) 1.0 不稳定型心绞痛 9(56.3) 11(73.3) 0.46 17(68.0) 12(70.6) 0.86 未知 1(6.3) 1(6.7) 1.0 1(4.0) 0(0.0) 1.0 血脂 总胆固醇/(mmol·L-1) 3.82±0.75 3.95±0.94 0.67 4.39±0.99 3.98±0.87 0.17 甘油三酯/(mmol·L-1) 1.53±0.70 2.20±1.15 0.06 1.19±0.45 2.35±1.09 0.001 HDL-C/(mmol·L-1) 1.20±0.26 0.83±0.15 < 0.001 1.36±0.30 0.86±0.11 < 0.001 LDL-C/(mmol·L-1) 2.18±0.63 2.23±0.76 0.85 2.64±0.87 2.47±0.69 0.49 他汀治疗 16(100.0) 15(100.0) 25(100.0) 17(100.0) 抗血小板/抗凝治疗 单联抗血小板治疗 1(6.3) 0(0.0) 1.0 1(4.0) 0(0.0) 1.0 DAPT 15(93.8) 15(100.0) 1.0 24(96.0) 16(94.1) 1.0 DAPT +口服抗凝药 0(0.0) 0(0.0) 0(0.0) 1(5.9) 0.41 CABG:冠脉旁路移植;LDL-C:低密度脂蛋白胆固醇。 表 2 入组患者的靶病变特征
Table 2. Target lesion characteristics of enrolled patients
处(%), X±S 靶病变特征 队列-1 队列-2 高HDL-C组(17处) 低HDL-C组(18处) P 高HDL-C组(28处) 低HDL-C组(19处) P 靶血管 左主干 0(0.0) 0(0.0) 0(0.0) 0(0.0) 前降支 10(58.8) 6(33.3) 0.18 16(57.1) 6(31.6) 0.09 回旋支 2(11.8) 2(11.1) 1.0 4(14.3) 4(21.1) 0.83 右冠脉 5(29.4) 10(55.6) 0.18 8(28.6) 9(47.4) 0.19 PCI术前冠脉造影 病变长度/mm 13.77±6.03 15.61±10.68 0.54 15.80±7.46 16.35±9.40 0.83 病变管腔直径/mm 2.91±0.41 2.75±0.53 0.33 2.87±0.49 2.86±0.49 0.99 最小管腔直径/mm 1.16±0.36 1.05±0.46 0.43 1.17±0.42 1.11±0.42 0.64 管腔狭窄率/% 59.82±12.86 61.61±15.40 0.71 59.21±13.08 61.44±13.95 0.59 球囊预扩张率/% 14(82.4) 14(77.8) 1.0 20(71.4) 15(78.9) 0.81 球囊直径/mm 2.25±0.24 2.21±0.26 0.71 2.34±0.31 2.40±0.26 0.53 最大扩张压力/atm 11.86±2.77 10.57±2.95 0.25 11.95±3.02 11.33±2.79 0.54 支架植入 BuMA supreme支架 10(58.8) 8(44.4) 0.51 12(42.9) 9(47.4) 0.76 支架直径/mm 3.22±0.36 3.03±0.41 0.15 3.18±0.41 3.20±0.38 0.88 支架长度/mm 24.29±6.78 23.28±6.69 0.66 25.43±7.25 26.63±8.33 0.60 最大扩张压力/atm 12.53±2.79 11.83±2.73 0.46 12.00±2.80 12.16±2.50 0.84 球囊后扩张率% 17(100.0) 14(77.8) 0.10 25(89.3) 17(89.5) 1 球囊直径/mm 3.50±0.48 3.36±0.53 0.43 3.50±0.43 3.54±0.46 0.75 最大扩张压力/atm 17.00±3.04 19.07±4.05 0.12 17.68±2.98 19.06±3.75 0.19 手术成功率/% 100 100 100 100 表 3 QCA结果
Table 3. Results of QCA
X±S 项目 队列-1 队列-2 高HDL-C组(n=16,L=17) 低HDL-C组(n=15,L=18) P 高HDL-C组(n=25,L=28) 低HDL-C组(n=17,L=19) P 支架内分析 PCI术后 病变管腔直径/mm 3.00±0.42 2.88±0.47 0.43 2.90±0.42 3.02±0.49 0.38 最小管腔直径/mm 2.65±0.37 2.58±0.45 0.61 2.57±0.37 2.65±0.46 0.51 管腔狭窄率/% 11.26±5.16 10.28±5.90 0.60 11.00±8.14 12.18±4.70 0.57 即刻血管直径获得值/mm 1.54±0.65 1.56±0.47 0.89 1.35±0.34 1.57±0.50 0.10 随访 病变管腔直径/mm 2.88±0.40 2.81±0.47 0.64 2.84±0.36 2.93±0.45 0.46 最小管腔直径/mm 2.60±0.39 2.51±0.45 0.54 2.50±0.40 2.59±0.43 0.49 管腔狭窄率/% 9.50±7.05 10.59±5.32 0.62 11.96±6.32 11.68±4.80 0.87 二元再狭窄率/% 0 0 0 0 晚期管腔丢失值/mm 0.06±0.26 0.09±0.19 0.73 0.06±0.24 0.06±0.18 0.93 病变节段内分析 PCI术后 病变管腔直径/mm 2.89±0.43 2.83±0.50 0.69 2.80±0.46 2.91±0.51 0.45 最小管腔直径/mm 2.39±0.55 2.28±0.49 0.55 2.26±0.46 2.46±0.53 0.19 管腔狭窄率/% 18.12±11.09 19.72±8.37 0.63 19.15±9.31 15.89±6.59 0.17 随访 病变管腔直径/mm 2.81±0.46 2.71±0.43 0.50 2.71±0.41 2.86±0.47 0.28 最小管腔直径/mm 2.35±0.50 2.24±0.51 0.54 2.26±0.43 2.42±0.46 0.23 管腔狭窄率/% 16.78±8.49 18.09±10.55 0.70 16.93±8.34 15.58±5.39 0.54 二元再狭窄率/% 0 0 0 0 晚期管腔丢失值/mm 0.09±0.28 0.05±0.28 0.66 0.00±0.28 0.04±0.18 0.61 表 4 OCT结果
Table 4. Results of OCT
X±S 项目 队列-1 队列-2 高HDL-C组(n=16,L=17) 低HDL-C组(n=15,L=18) P 高HDL-C组(n=25,L=28) 低HDL-C组(n=17,L=19) P 随访 支架小梁血管内膜覆盖率/% 84.53±12.51 72.97±15.52 0.021 74.47±18.62 79.37±22.32 0.42 支架小梁内膜未覆盖率>30%的靶病变比例/% 11.8 44.4 0.06 39.3 31.6 0.59 平均血管内膜增生面积/mm2 0.62±0.38 0.38±0.15 0.024 0.53±0.23 0.59±0.41 0.56 血管内膜增生体积阻塞百分率/% 7.71±5.08 4.87±1.48 0.039 6.34±2.75 6.71±4.91 0.74 平均支架贴壁不良面积/mm2 0.04±0.07 0.03±0.05 0.65 0.02±0.06 0.03±0.05 0.73 HS评分 31.13±25.81 54.79±30.69 0.019 51.23±37.44 42.11±45.87 0.46 支架区域长度/mm 26.70±11.03 27.57±14.30 0.84 26.85±8.66 36.19±15.58 0.025 平均支架面积/mm2 8.42±1.62 7.72±2.37 0.32 8.58±2.29 9.00±2.83 0.58 最小支架面积/mm2 7.05±1.52 6.12±2.14 0.15 6.98±2.12 7.40±2.78 0.55 平均管腔面积/mm2 7.95±1.76 7.54±2.35 0.56 8.22±2.21 8.57±2.77 0.63 最小管腔面积/mm2 6.55±1.45 5.72±2.01 0.18 6.44±2.00 6.94±2.83 0.49 支架小梁水平分析 支架小梁数量 308.47±146.33 312.44±169.54 0.94 293.71±95.39 418.68±222.73 0.05 未覆盖的支架小梁比例/% 15.47±12.51 27.03±15.52 0.021 25.53±18.62 20.63±22.32 0.42 贴壁并覆盖良好的支架小梁比例/% 83.03±13.28 71.47±14.92 0.021 73.40±18.77 77.94±23.33 0.47 贴壁不良的支架小梁比例/% 1.00±1.59 1.18±1.59 0.75 0.67±1.42 1.00±1.91 0.50 贴壁不良并覆盖良好的支架小梁比例/% 0.95±1.53 1.06±1.57 0.83 0.65±1.43 0.98±1.83 0.49 贴壁不良并未覆盖的支架小梁比例/% 0.05±0.21 0.11±0.40 0.57 0.02±0.12 0.02±0.10 1.00 表 5 相关性分析结果
Table 5. Results of correlation analysis
项目 队列-1 队列-2 r P r P 性别 0.036 0.84 -0.074 0.62 年龄a) -0.085 0.63 0.177 0.24 基线HDL-C水平≥1.0 mmol/L 0.430 0.01 -0.185 0.21 糖尿病 0.053 0.76 -0.041 0.78 BuMA支架的使用 0.289 0.09 -0.180 0.23 不稳定型心绞痛 -0.200 0.25 -0.139 0.35 球囊后扩张 0.196 0.26 -0.290 0.05 a)使用Pearson检验;其他指标使用Spearman检验。 -
[1] Lewis GF, Rader DJ. New insights into the regulation of HDL metabolism and reverse cholesterol transport[J]. Circ Res, 2005, 96(12): 1221-1232. doi: 10.1161/01.RES.0000170946.56981.5c
[2] Toth PP. High-density lipoprotein and cardiovascular risk[J]. Circulation, 2004, 109(15): 1809-1812. doi: 10.1161/01.CIR.0000126889.97626.B8
[3] Emerging Risk Factors Collaboration, Di Angelantonio E, Sarwar N, et al. Major lipids, apolipoproteins, and risk of vascular disease[J]. JAMA, 2009, 302(18): 1993-2000. doi: 10.1001/jama.2009.1619
[4] Joint committee for guideline revision. 2016 Chinese guidelines for the management of dyslipidemia in adults[J]. J Geriatr Cardiol, 2018, 15(1): 1-29.
[5] Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association[J]. Circulation, 2015, 131(4): e29-e322.
[6] Chen W, Habraken TC, Hennink WE, et al. Polymer-free drug-eluting stents: An overview of coating strategies and comparison with polymer-coated drug-eluting stents[J]. Bioconjug Chem, 2015, 26(7): 1277-1288. doi: 10.1021/acs.bioconjchem.5b00192
[7] Ferns GA, Avades TY. The mechanisms of coronary restenosis: insights from experimental models[J]. Int J Exp Pathol, 2000, 81(2): 63-88.
[8] Saleh YE, Gepreel MA, Allam NK. Functional nanoarchitectures for enhanced drug eluting stents[J]. Sci Rep, 2017, 7: 40291. doi: 10.1038/srep40291
[9] Nofer JR, Kehrel B, Fobker M, et al. HDL and arteriosclerosis: beyond reverse cholesterol transport[J]. Atherosclerosis, 2002, 161(1): 1-16.
[10] von Birgelen C, Asano T, Amoroso G, et al. First-in-man randomised comparison of the BuMA Supreme biodegradable polymer sirolimus-eluting stent versus a durable polymer zotarolimus-eluting coronary stent: the PIONEER trial[J]. EuroIntervention, 2018, 13(17): 2026-2035. doi: 10.4244/EIJ-D-17-00462
[11] Xu B, Gao R, Yang Y, et al. Biodegradable polymer-based sirolimus-eluting stents with differing elution and absorption kinetics: The PANDA Ⅲ trial[J]. J Am Coll Cardiol, 2016, 67(19): 2249-2258. doi: 10.1016/j.jacc.2016.03.475
[12] Qian J, Zhang YJ, Xu B, et al. Optical coherence tomography assessment of a PLGA-polymer with electro-grafting base layer versus a PLA-polymer sirolimus-eluting stent at three-month follow-up: the BuMA-OCT randomised trial[J]. EuroIntervention, 2014, 10(7): 806-814.
[13] Ding NI, Pacetti SD, Tang FW, et al. XIENCE VTM stent design and rationale[J]. J IntervCardiol, 2010, 22(s1): S18-S27.
[14] Perkins LEL, Boeke Purkis KH, Wang Q, et al. XIENCE VTM everolimus-eluting coronary stent system: A preclinical assessment[J]. J Interv Cardiol, 2010, 22(s1): S28-S40.
[15] Sheiban I, Villata G, Bollati M, et al. Next-generation drug-eluting stents in coronary artery disease: focus on everolimus-eluting stent(Xience V)[J]. Vasc Health Risk Manag, 2008, 4(1): 31-38. doi: 10.2147/vhrm.2008.04.01.31
[16] Suwannasom P, Onuma Y, Benit E, et al. Evaluation of vascular healing of polymer-free sirolimus-eluting stents in native coronary artery stenosis: a serial follow-up at three and six months with optical coherence tomography imaging[J]. EuroIntervention, 2016, 12(5): e574-e583. doi: 10.4244/EIJV12I5A97
[17] Garcia-Garcia HM, Muramatsu T, Nakatani S, et al. Serial optical frequency domain imaging in STEMI patients: the follow-up report of TROFI study[J]. Eur Heart J Cardiovasc Imaging, 2014, 15(9): 987-995. doi: 10.1093/ehjci/jeu042
[18] Sabate M, Windecker S, Iniguez A, et al. Everolimus-eluting bioresorbable stent vs. durable polymer everolimus-eluting metallic stent in patients with ST-segment elevation myocardial infarction: results of the randomized ABSORB ST-segment elevation myocardial infarction-TROFI Ⅱ trial[J]. Eur Heart J, 2016, 37(3): 229-240. doi: 10.1093/eurheartj/ehv500
[19] Suwannasom P, Sotomi Y, Corti R, et al. First-in-man six-month results of a surface-modified coronary stent system in native coronary stenosis[J]. EuroIntervention, 2017, 12(17): 2118-2127. doi: 10.4244/EIJ-D-16-00975
[20] Catapano AL, Graham I, De Backer G, et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias[J]. Eur Heart J, 2016, 37(39): 2999-3058.
[21] Finn AV, Joner M, Nakazawa G, et al. Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization[J]. Circulation, 2007, 115(18): 2435-2441. doi: 10.1161/CIRCULATIONAHA.107.693739
[22] Asano T, Jin Q, Katagiri Y, et al. A randomised comparison of healing response between the BuMA Supreme stent and the XIENCE stent at one-month and two-month follow-up: PIONEER-Ⅱ OCT randomised controlled trial[J]. EuroIntervention, 2018, 14(12): e1306-e1315. doi: 10.4244/EIJ-D-18-00461
[23] Suh Y, Kim BK, Shin DH, et al. Impact of statin treatment on strut coverage after drug-eluting stent implantation[J]. Yonsei Med J, 2015, 56(1): 45-52. doi: 10.3349/ymj.2015.56.1.45
[24] Shah PK, Amin J. Low high density lipoprotein level is associated with increased restenosis rate after coronary angioplasty[J]. Circulation, 1992, 85(4): 1279-1285. doi: 10.1161/01.CIR.85.4.1279
[25] Topakian R, Sonnberger M, Nussbaumer K, et al. Postprocedural high-density lipoprotein cholesterol predicts carotid stent patency at 1 year[J]. Eur J Neurol, 2008, 15(2): 179-184. doi: 10.1111/j.1468-1331.2007.02026.x
[26] Ghazzal ZB, Dhawan SS, Sheikh A, et al. Usefulness of serum high-density lipoprotein cholesterol level as an independent predictor of one-year mortality after percutaneous coronary interventions[J]. Am J Cardiol, 2009, 103(7): 902-906. doi: 10.1016/j.amjcard.2008.11.053
[27] Seetharam D, Mineo C, Gormley AK, et al. High-density lipoprotein promotes endothelial cell migration and reendothelialization via scavenger receptor-B type I[J]. Circ Res, 2006, 98(1): 63-72. doi: 10.1161/01.RES.0000199272.59432.5b
[28] Tamagaki T, Sawada S, Imamura H, et al. Effects of high-density lipoproteins on intracellular pH and proliferation of human vascular endothelial cells[J]. Atherosclerosis, 1996, 123(1-2): 73-82.
[29] Vanags LZ, Tan J, Galougahi KK, et al. Apolipoprotein A-I reduces in-stent restenosis and platelet activation and alters neointimal cellular phenotype[J]. JACC Basic Transl Sci, 2018, 3(2): 200-209.
[30] Vanags LZ, Tan J, Santos M, et al. Plasma activated coating immobilizes apolipoprotein A-I to stainless steel surfaces in its bioactive form and enhances biocompatibility[J]. Nanomedicine, 2017, 13(7): 2141-2150.
-




 下载:
下载: