Chinese Expert Consensus on the use of GLP-1RAs in patients with type 2 diabetes mellitus complicated by arteriosclerotic cardiovascular disease
-
摘要: 胰高糖素样肽-1受体激动剂(GLP-1RAs)除具有很强的降糖作用外,也具有很强的心血管保护作用。随着心血管结局研究结果的公布,GLP-1RAs也被证实可显著改善2型糖尿病患者合并动脉粥样硬化性心脏病的远期风险。本共识针对GLP-1RAs药理学、心血管保护机制、循证医学证据、临床应用建议、不良反应及注意事项等临床问题给出具体的推荐意见。
-
关键词:
- 胰高血糖素样肽-1受体激动剂 /
- 2型糖尿病 /
- 动脉粥样硬化性心血管疾病
Abstract: Glucagon like peptide-1 receptor agonists (GLP-1RAs) not only have strong hypoglycemic effects, but also have strong cardiovascular protective effects. With the announcement of cardiovascular outcomes, GLP-1RAs have been proved to improve the long-term risk of type 2 diabetes patients with atherosclerotic heart disease significantly.This consensus provides specific recommendations on clinical issues such as GLP-1RAs pharmacology, cardiovascular protection mechanisms, evidence-based medical evidence, clinical application recommendations, adverse reactions, and precautions. -

-
表 1 GLP-1RAs分子结构和药代动力学特点
Table 1. Molecular structure and pharmacokinetic characteristics of GLP-1RAs
药物 分子结构 与人GLP-1
同源性/%作用时间 半衰期 给药频率 艾塞那肽 exendin-4 53 短效 2.4 h 每日2次 贝那鲁肽 重组人GLP-1 100 短效 11 min 每日3次 利司那肽 改良的exendin-4 50 短效 2~5 h 每日1次 利拉鲁肽 改良的人GLP-1 97 长效 13 h 每日1次 艾塞那肽周制剂 exendin-4 53 长效 2.4 h(缓释剂) 每周1次 阿必鲁肽 改良的人GLP-1 97 长效 4~5 d 每周1次 度拉糖肽 改良的人GLP-1 90 长效 4.7 d 每周1次 司美格鲁肽 改良的人GLP-1 94 长效 165 h 每周1次 表 2 GLP-1RAs在T2DM合并ASCVD中应用的主要随机临床试验汇总(一)
Table 2. Summary of CVOT on the cardiovascular application of GLP-1RAs
药物 主要心血管事件 全因死亡 心血管死亡 非致死性卒中 心力衰竭住院率 肾脏复合结局 阿必鲁肽 Harmony Outcomes 利司那肽 ELIXA 度拉糖肽 REWIND REWIND REWIND 艾塞那肽 EXSCEL 利拉鲁肽 LEADER LEADER LEADER LEADER 司美格鲁肽 SUSTAIN6-13 PIONEER 6 PIONEER 6 SUSTAIN-6 SUSTAIN-6 Efpeglenatide AMPLITUDE-O AMPLITUDE-O AMPLITUDE-O 表 3 GLP-1RAs在T2DM合并ASCVD中应用的主要随机临床试验汇总(二)
Table 3. Summary of CVOT on the cardiovascular application of GLP-1RAs
试验 ELIXA LEADER SUSTAIN-6 EXSCEL REWIND PIONEER-6 Hamony AMPLITUDE-O 药物 利司那肽 利拉鲁肽 司美格鲁肽皮下 艾塞那肽 度拉糖肽 司美格鲁肽口服 阿必鲁肽 Efpeglenatide 入组受试者/例 6 068 9 340 3 297 1 4752 9 901 3 183 9 463 4 076 随访时间/年 2.1 3.8 2.1 3.2 5.4 1.3 2.3 2 合并ASCVD/% 100 81 72 73 31 85 70 89.6 基线心力衰竭/% 22 18 24 16 9 未报道 20 18.1 糖尿病病史/年 9.3 12.8 13.9 12.0 9.5 14.9 14.2 15.4 基线HbA1c/% 7.7 8.7 8.7 8.0 7.2 8.2 8.4 8.91 他汀应用率/% 93 72 73 74 66 85 84 80.8 主要终点事件/HR(95%CI) 1.02
(0.89~1.17)0.87
(0.78~0.97)0.74
(0.58~0.95)0.91
(0.83~1.00)0.88
(0.79~0.99)0.79
(0.57~1.11)0.78
(0.68~0.90)0.73
(0.58~0.92)主要终点事件发生数量 844例 1 302例 254例 839例 2.35例/100例 136例 766例 314例 心血管死亡率/HR(95%CI) 0.98
(0.78~1.22)0.78
(0.66~0.93)0.98
(0.65~1.48)0.88
(0.76~1.02)0.91
(0.87~1.06)0.49
(0.27~0.92)0.93
(0.73~1.19)0.71
(0.59~0.87)心肌梗死发生率/HR(95%CI) 1.03
(0.87~1.22)0.86
(0.73~1.00)0.74
(0.51~1.08)0.97
(0.85~1.10)0.96
(0.79~1.15)1.18
(0.73~1.90)0.75
(0.61~0.90)0.75
(0.54~1.05)卒中发生率/HR(95%CI) 1.12
(0.79~1.58)0.86
(0.71~1.06)0.61
(0.38~0.99)0.85
(0.70~1.03)0.76
(0.62~0.94)0.74
(0.35~1.57)0.86
(0.66~1.14)0.74
(0.47~1.17)全因死亡率/HR(95%CI) 0.94
(0.78~1.13)0.85
(0.74~0.97)1.05
(0.74~1.50)0.86
(0.77~0.97)0.90
(0.80~1.01)0.51
(0.31~0.84)0.95
(0.79~1.16)0.78
(0.58~1.06)心力衰竭住院率/HR(95%CI) 0.96
(0.75~1.23)0.87
(0.73~1.05)0.86
(0.48~1.55)0.94
(0.78~1.13)0.93
(0.77~1.12)1.11
(0.77~1.61)0.85
(0.70~1.04)0.61
(0.380.98)表 4 GLP-1RAs治疗CVD的临床应用建议
Table 4. Clinical application recommendations for GLP-1RAs in the treatment of ASCVD
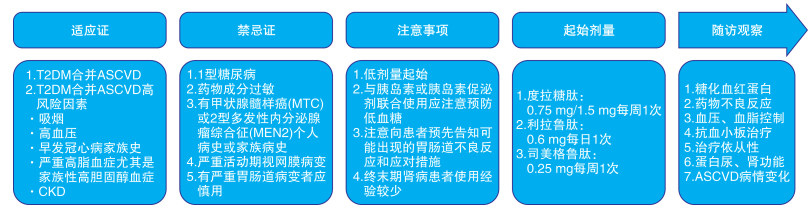
建议 推荐级别 证据等级 T2DM合并ASCVD患者推荐起始使用具有明确心血管获益的GLP-1RAs,不需考虑患者HbA1c水平和二甲双胍是否正在使用 Ⅰ A T2DM合并ASCVD高危因素但未确诊ASCVD患者推荐应用具有明确心血管获益的GLP-1RAs,其中度拉糖肽可用于主要心血管病变的一级预防 Ⅱa B 推荐级别:Ⅰ级指已证实和(或)一致公认有益、有用和有效的操作或治疗,推荐使用;Ⅱ级指有用和(或)有效的证据尚有矛盾或存在不同观点的操作或治疗;Ⅱa级指有关证据/观点倾向于有用和(或)有效,应用这些操作或治疗是合理的,应当考虑使用;Ⅱb级指有关证据/观点尚不能被充分证明有用和(或)有效,可以考虑使用;Ⅲ级指已证实和(或)一致公认无用和(或)无效,并对一些病例可能有害的操作或治疗,不推荐使用。证据水平:A级资料来源于多项随机对照研究或荟萃分析;B级资料来源于单项随机对照研究或大型非随机对照研究;C级资料来源于专家共识和(或)小型研究、回顾性分析、注册研究。 表 5 GLP-1RAs的用法与用量
Table 5. Usage and dosage of GLP-1RAs
药物 规格 初始剂量 剂量调整/目标剂量 药物的供应形式 是否配
有针头利拉鲁肽 3 mL:18 mg 0.6 mg每日1次(可在任何时间给药) 可每周增加1次剂量,1周后增至1.2 mg每日1次。如需进一步控制血糖,可继续增至1.8 mg每日1次 1支注射笔有3个不同的可程序化调节剂量(0.6、1.2和1.8 mg)。注射笔可多次使用,针头为一次性 否 注射用司美格鲁肽 1.34 mg/mL,1.5 mL
1.34 mg/mL,3 mL0.25 mg每周1次(可在任何时间给药) 可每4周增加1次剂量,4周后增至0.5 mg每周1次。如需进一步控制血糖,可继续增至1.0 mg每周1次 提供2种不同的注射笔,每支笔有2个可程序化调节的剂量:一支笔允许使用0.25和0.5 mg的剂量,另一支笔允许使用1.0 mg的剂量。注射笔可多次使用,针头为一次性 否 度拉糖肽 1.5 mg:0.5 mL 1.5 mg每周1次 注射笔为一次性 是 -
[1] 国家心血管病中心. 中国心血管健康与疾病报告2021[M]. 北京: 科学出版社. 2022.
[2] Verdich C, Flint A, Gutzwiller JP, et al. A meta-analysis of the effect of glucagon-like peptide-1(7-36) amide on ad libitum energy intake in humans[J]. J Clin Endocrinol Metab, 2001, 86(9): 4382-4389.
[3] Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes[J]. N Engl J Med, 2016, 375(4): 311-322. doi: 10.1056/NEJMoa1603827
[4] Marso SP, Bain SC, Consoli A, et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes[J]. N Engl J Med, 2016, 375(19): 1834-1844. doi: 10.1056/NEJMoa1607141
[5] Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes(REWIND): a double-blind, randomised placebo-controlled trial[J]. Lancet, 2019, 394(10193): 121-130. doi: 10.1016/S0140-6736(19)31149-3
[6] Zhao X, Wang M, Wen Z, et al. GLP-1 Receptor Agonists: Beyond Their Pancreatic Effects[J]. Front Endocrinol, 2021, 12: 721135. doi: 10.3389/fendo.2021.721135
[7] Song G, Yang D, Wang Y, et al. Human GLP-1 receptor transmembrane domainstructureincomplexwithallostericmodulators[J]. Nature, 2017, 546(7657): 312-315. doi: 10.1038/nature22378
[8] Winquist RJ, Gribkoff VK. Cardiovascular effects of GLP-1 receptor agonism[J]. Adv Pharmacol, 2022, 94: 213-254.
[9] American Diabetes Association. 9. Pharmacologic approaches toglycemic treatment: standards of medical care in diabetes-2021[J]. Diabetes Care, 2021, 44(Suppll): S111-S124.
[10] 中华医学会糖尿病学分会. 中国2型糖尿病防治指南(2020年版)[J]. 中华糖尿病杂志, 2021, 13(4): 315-409. https://www.cnki.com.cn/Article/CJFDTOTAL-HBYX202112018.htm
[11] 纪立农. 胰高血糖素样肽1受体激动剂周制剂中国证据与专家指导建议[J]. 中国糖尿病杂志, 2022, 30(6): 7. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGTL202206016.htm
[12] Berndt J, Ooi SL, Pak SC. What Is the Mechanism Driving the Reduction of Cardiovascular Events from Glucagon-like Peptide-1 Receptor Agonists?-A Mini Review[J]. Molecules, 2021, 26(16): 4822. doi: 10.3390/molecules26164822
[13] Samson SL, Vellanki P, Blonde L, et al. American Association of Clinical Endocrinology Consensus Statement: Comprehensive Type 2 Diabetes Management Algorithm-2023 Update[J]. Endocr Pract, 2023, 29(5): 305-340. doi: 10.1016/j.eprac.2023.02.001
[14] 中华医学会内分泌学分会, 中华医学会糖尿病学分会. 胰高糖素样肽-1(GLP-1)受体激动剂用于治疗2型糖尿病的临床专家共识[J]. 中华内科杂志, 2020, 59(11): 836-846.
[15] Frison V, Simioni N, Marangoni A, et al. Clinical Impact of 5 Years of Liraglutide Treatment on Cardiovascular Risk Factors in Patients with Type 2 Diabetes Mellitus in a Real-Life Setting in Italy: An Observational Study[J]. Diabetes Ther, 2018, 9(6): 2201-2208. doi: 10.1007/s13300-018-0503-4
[16] Dandona P, Ghanim H, Chaudhuri A. Incretins: Beyond type 2 diabetes[J]. Diabetes Obes Metab, 2018, 20 Suppl 1: 59-67.
[17] Secher A, Jelsing J, Baquero AF, et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss[J]. J Clin Invest, 2014, 124(10): 4473-4488. doi: 10.1172/JCI75276
[18] Sun F, Wu S, Guo S, et al. Impact of GLP-1 receptor agonists on blood pressure, heart rate and hypertension among patients with type 2 diabetes: A systematic review and network meta-analysis[J]. Diabetes Res Clin Pract, 2015, 110(1): 26-37. doi: 10.1016/j.diabres.2015.07.015
[19] Liakos CI, Papadopoulos DP, Sanidas EA, et al. Blood Pressure-Lowering Effect of Newer Antihyperglycemic Agents(SGLT-2 Inhibitors, GLP-1 Receptor Agonists, and DPP-4 Inhibitors)[J]. Am J Cardiovasc Drugs, 2021, 21(2): 123-137. doi: 10.1007/s40256-020-00423-z
[20] Zinman B, Schmidt WE, Moses A, et al. Achieving a clinically relevant composite outcome of an HbA1c of < 7% without weight gain or hypoglycaemia in type 2 diabetes: a meta-analysis of the liraglutide clinical trial programme[J]. Diabetes Obes Metab, 2012, 14(1): 77-82. doi: 10.1111/j.1463-1326.2011.01493.x
[21] Rigato M, Avogaro A, Vigili de Kreutzenberg S, Fadini GP. Effects of Basal Insulin on Lipid Profile Compared to Other Classes of Antihyperglycemic Agents in Type 2 Diabetic Patients[J]. J Clin Endocrinol Metab, 2020, 105(7): dgaa178.
[22] Pignatelli P, Baratta F, Buzzetti R, et al. The Sodium-Glucose Co-Transporter-2(SGLT2) Inhibitors Reduce Platelet Activation and Thrombus Formation by Lowering NOX2-Related Oxidative Stress: A Pilot Study[J]. Antioxidants, 2022, 11(10): 1878. doi: 10.3390/antiox11101878
[23] Loganathan J, Cohen AC, Kaloupis GM, et al. A pilot clinical study to Evaluate Liraglutide-mediated Anti-platelet activity in patients with type-2 Diabetes(ELAID study)[J]. J Diabetes Complications, 2022, 36(5): 108188. doi: 10.1016/j.jdiacomp.2022.108188
[24] Lee YS, Jun HS. Anti-Inflammatory Effects of GLP-1-Based Therapies beyond Glucose Control[J]. Mediators Inflamm, 2016: 3094642.
[25] Xia J, Li Q, Liu Y, et al. A GLP-1 Analog Liraglutide Reduces Intimal Hyperplasia After Coronary Stent Implantation via Regulation of Glycemic Variability and NLRP3 Inflammasome/IL-10 Signaling in Diabetic Swine[J]. Front Pharmacol, 2020, 11: 372. doi: 10.3389/fphar.2020.00372
[26] Jensen JK, Zobel EH, von Scholten BJ, et al. Effect of 26 Weeks of Liraglutide Treatment on Coronary Artery Inflammation in Type 2 Diabetes Quantified by[64Cu]Cu-DOTATATE PET/CT: Results from the LIRAFLAME Trial[J]. Front Endocrinol(Lausanne), 2021, 12: 790405. doi: 10.3389/fendo.2021.790405
[27] Tsai TH, Lee CH, Cheng CI, et al. Liraglutide Inhibits Endothelial-to-Mesenchymal Transition and Attenuates Neointima Formation after Endovascular Injury in Streptozotocin-Induced Diabetic Mice[J]. Cells, 2019, 8(6): 589. doi: 10.3390/cells8060589
[28] Bruen R, Curley S, Kajani S, et al. Liraglutide Attenuates Preestablished Atherosclerosis in Apolipoprotein E-Deficient Mice via Regulation of Immune Cell Phenotypes and Proinflammatory Mediators[J]. J Pharmacol Exp Ther, 2019, 370(3): 447-458. doi: 10.1124/jpet.119.258343
[29] Rizzo M, Chandalia M, Patti AM, et al. Liraglutide decreases carotid intima-media thickness in patients with type 2 diabetes: 8-month prospective pilot study[J]. Cardiovasc Diabetol, 2014, 13: 49. doi: 10.1186/1475-2840-13-49
[30] Tuttle KR, Lakshmanan MC, Rayner B, et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease(AWARD-7): a multicentre, open-label, randomised trial[J]. Lancet Diabetes Endocrinol, 2018, 6(8): 605-617. doi: 10.1016/S2213-8587(18)30104-9
[31] Hirose N, Tsujimoto N, Katayose T, et al. Utilization of glucagon-like peptide-1 receptor agonists and changes in clinical characteristics in patients with type 2 diabetes by chronic kidney disease stage in Japan: A descriptive observational study using a nationwide electronic medical records database[J]. Diabetes Obes Metab, 2022, 24(3): 486-498. doi: 10.1111/dom.14600
[32] Mancini GBJ, O'Meara E, Zieroth S, et al. 2022 Canadian CardiovascularSociety Guideline for Use of GLP-1 Receptor Agonists and SGLT2 Inhibitors forCardiorenalRiskReductioninAdults[J]. Can J Cardiol, 2022, 38(8): 1153-1167. doi: 10.1016/j.cjca.2022.04.029
[33] Martens P, Mathieu C, Vanassche T. The use of glucagon-like-peptide-1 receptor agonist in the cardiology practice[J]. Acta Cardiol, 2023, 78(5): 552-564. doi: 10.1080/00015385.2022.2076307
[34] Hernandez AF, Green JB, Janmohamed S, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease(Harmony Outcomes): a double-blind, randomised placebo-controlled trial[J]. Lancet, 2018, 392(10157): 1519-1529. doi: 10.1016/S0140-6736(18)32261-X
[35] Husain M, Birkenfeld AL, Donsmark M, et al. Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes[J]. N Engl J Med, 2019, 381(9): 841-851. doi: 10.1056/NEJMoa1901118
[36] Gerstein HC, Sattar N, Rosenstock J, et al. Cardiovascular and Renal Outcomes with Efpeglenatide in Type 2 Diabetes[J]. N Engl J Med, 2021, 385(10): 896-907. doi: 10.1056/NEJMoa2108269
[37] Svanström H, Ueda P, Melbye M, et al. Use of liraglutide and risk major cardiovascular events: a register-based cohort study in Denmark and Swede[J]Lancet Diabetes Endocrinol, 2019, 7(2): 106-114. doi: 10.1016/S2213-8587(18)30320-6
[38] Trevisan M, Fu EL, Szummer K, et al. Glucagon-like peptide-1 receptor agonists and the risk of cardiovascular events in diabetes patients surviving an acute myocardial infarction[J]. Eur Heart J Cardiovasc Pharmacother, 2021, 7(2): 104-111. doi: 10.1093/ehjcvp/pvaa004
[39] Wong SY, Lee A, Sia A, et al. Effects of Glucagon-Like Peptide-1 Receptor Agonist(GLP-1RA)on Cardiac Structure and Function: A Systematic Review and Meta-Analysis of Randomized-Controlled Trials[J]. Cardiovasc Drugs Ther, 2022.
[40] Alfayez OM, Almutairi AR, Aldosari A, et al. Update on Cardiovascular Safety of Incretin-Based Therapy in Adults With Type 2 Diabetes Mellitus: A Meta-Analysis of Cardiovascular Outcome Trials[J]. Can J Diabetes, 2019, 43(7): 538-545. e2. doi: 10.1016/j.jcjd.2019.04.003
[41] Sattar N, Lee M, Kristensen SL, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials[J]. Lancet Diabetes Endocrinol, 2021, 9(10): 653-662. doi: 10.1016/S2213-8587(21)00203-5
[42] Hulst AH, Visscher MJ, Cherpanath T, et al. Effects of Liraglutide on Myocardial Function After Cardiac Surgery: A Secondary Analysis of the Randomised Controlled GLOBE Trial[J]. J Clin Med, 2020, 9(3): 673. doi: 10.3390/jcm9030673
[43] Buse JB, Wexler DJ, Tsapas A, et al. 2019 Update to: Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association(ADA)and the European Association for the Study of Diabetes(EASD)[published correction appears in Diabetes Care. 2020 Jul; 43(7): 1670][J]. Diabetes Care, 2020, 43(2): 487-493.
[44] Zelniker TA, Wiviott SD, Raz I, et al. Comparison of the Effects of Glucagon-Like Peptide Receptor Agonists and Sodium-Glucose Cotransporter 2 Inhibitors for Prevention of Major Adverse Cardiovascular and Renal Outcomes in Type 2 Diabetes Mellitus[J]. Circulation, 2019, 139(17): 2022-2031. doi: 10.1161/CIRCULATIONAHA.118.038868
[45] Ludvik B, Frías JP, Tinahones FJ, et al. Dulaglutide as add-on therapyto SGLT2 inhibitors in patients with inadequately controlled type 2diabetes(AWARD-10): a 24-week, randomised, double-blind, placebo-controlled trial[J]. Lancet Diabetes Endocrinol, 2018, 6(5): 370-381. doi: 10.1016/S2213-8587(18)30023-8
[46] Frías JP, Guja C, Hardy E, et al. Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy(DURATION-8): a 28 week, multicentre, double-blind, phase 3, randomised controlled trial[J]. Lancet Diabetes Endocrinol, 2016, 4(12): 1004-1016. doi: 10.1016/S2213-8587(16)30267-4
[47] Zinman B, Bhosekar V, Busch R, et al. Semaglutide once weekly as add-on to SGLT-2 inhibitor therapy in type 2 diabetes(SUSTAIN 9): a randomised, placebo-controlled trial[J]. Lancet Diabetes Endocrinol, 2019, 7(5): 356-367. doi: 10.1016/S2213-8587(19)30066-X
[48] Wright AK, Carr MJ, Kontopantelis E, et al. Primary Prevention of Cardiovascular and Heart Failure Events With SGLT2 Inhibitors, GLP-1 Receptor Agonists, and Their Combination in Type 2 Diabetes[J]. Diabetes Care, 2022, 45(4): 909-918. doi: 10.2337/dc21-1113
[49] Dave CV, Kim SC, Goldfine AB, et al. Risk of Cardiovascular Outcomes in Patients With Type 2 Diabetes After Addition of SGLT2 Inhibitors Versus Sulfonylureas to Baseline GLP-1RA Therapy[J]. Circulation, 2021, 143(8): 770-779. doi: 10.1161/CIRCULATIONAHA.120.047965
[50] Fadah K, Alashi A, Deoker A. The Enhanced Cardiac Outcome of Conjugated SGLT2 Inhibitors and GLP-1RA Therapy in Diabetic Patients[J]. Curr Cardiol Rep, 2022, 24(1): 17-22. doi: 10.1007/s11886-021-01619-8
[51] Azebu LM. The FDA's risk/benefit calculus in the approvals of Qsymia and Belviq: treating an obesity epidemic while avoiding another fen-phen[J]. Food Drug Law J, 2014, 69(1): 87-iii.
[52] Wilding JPH, Batterham RL, Calanna S, et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity[J]. N Engl J Med, 2021, 384(11): 989-1002. doi: 10.1056/NEJMoa2032183
[53] Zhu Y, Xu J, Zhang D, et al. Efficacy and Safety of GLP-1 Receptor Agonists in Patients With Type 2 Diabetes Mellitus and Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis[J]. Front Endocrinol(Lausanne), 2021, 12: 769069. doi: 10.3389/fendo.2021.769069
[54] Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in Patients with Type 2 Diabetes and Acute Coronary Syndrome[J]. N Engl J Med, 2015, 373(23): 2247-2257. doi: 10.1056/NEJMoa1509225
[55] Holman RR, Bethel MA, Mentz RJ, et al. Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes[J]. N Engl J Med, 2017, 377(13): 1228-1239. doi: 10.1056/NEJMoa1612917
-





 下载:
下载: