Research progress on the targeted regulatory mechanism of miRNA in diabetic cardiomyopathy
-
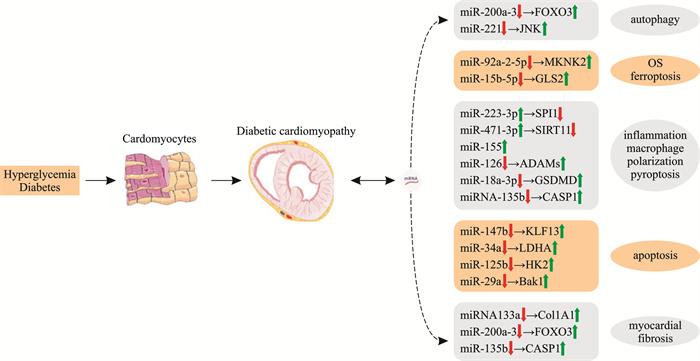
摘要: 糖尿病心肌病是在糖尿病存在的情况下出现的心肌收缩和(或)舒张功能障碍,严重危害人类健康。虽然对糖尿病心肌病的发病机制进行了广泛的研究,但其调控机制尚不明确。近年来的研究发现,miRNA特异性结合miRNA分子的3’-非翻译区的碱基序列,参与靶标的调控,在糖尿病心肌病的发生发展中发挥重要作用。本文就miRNA在糖尿病心肌病发病中的重要作用进行综述,以期为今后的研究提供参考。Abstract: Diabetic cardiomyopathy(DCM) is a systolic or diastolic myocardial dysfunction in diabetes, which seriously endangers human health. It is still unclear although the pathogenesis of DCM has been extensively studied. Recent studies have found that miRNA specifically binds to the base sequence of the 3'-untranslated region of mRNA molecules, participates in the regulation of targets, and plays an important role in the occurrence and development of DCM. This article reviews the important role of miRNA in the pathogenesis of DCM, in order to provide a reference for future research.
-
Key words:
- diabetic cardiomyopathy /
- microRNA /
- 3′-UTR /
- ferroptosis /
- macrophage polarization /
- pyroptosis /
- epigenetics
-

-
[1] 刘明波, 何新叶, 杨晓红, 等. 《中国心血管健康与疾病报告2023》要点解读[J]. 中国心血管杂志, 2024, 29(4): 305-324.
[2] Dunlay SM, Givertz MM, Aguilar D, et al. Type 2 diabetes mellitus and heart failure: a scientific statement from the American heart association and the heart failure society of America: this statement does not represent an update of the 2017 ACC/AHA/HFSA heart failure guideline update[J]. Circulation, 2019, 140(7): e294-e324.
[3] Seferović PM, Paulus WJ, Rosano G, et al. Diabetic myocardial disorder. A clinical consensus statement of the Heart Failure Association of the ESC and the ESC Working Group on Myocardial & Pericardial Diseases[J]. Eur J Heart Fail, 2024, 26(9): 1893-1903.
[4] Tan Y, Zhang ZG, Zheng C, et al. Mechanisms of diabetic cardiomyopathy and potential therapeutic strategies: preclinical and clinical evidence[J]. Nat Rev Cardiol, 2020, 17: 585-607. doi: 10.1038/s41569-020-0339-2
[5] Zhao X, Liu S, Wang X, et al. Diabetic cardiomyopathy: Clinical phenotype and practice[J]. Front Endocrinol(Lausanne), 2022, 13: 1032268. doi: 10.3389/fendo.2022.1032268
[6] Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene Lin-4 encodes small RNAs with antisense complementarity to Lin-14[J]. Cell, 1993, 75(5): 843-854. doi: 10.1016/0092-8674(93)90529-Y
[7] Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets[J]. Cell, 2005, 120(1): 15-20. doi: 10.1016/j.cell.2004.12.035
[8] Shang RF, Lee S, Senavirathne G, et al. MicroRNAs in action: biogenesis, function and regulation[J]. Nat Rev Genet, 2023, 24: 816-833.
[9] He XY, Kuang GY, Wu YR, et al. Emerging roles of exosomal miRNAs in diabetes mellitus[J]. Clin Transl Med, 2021, 11(6): e468. doi: 10.1002/ctm2.468
[10] Ma XZ, Mei S, Wuyun Q, et al. Epigenetics in diabetic cardiomyopathy[J]. Clin Epigenetics, 2024, 16(1): 52. doi: 10.1186/s13148-024-01667-1
[11] Hou J, Liang WY, Xiong SQ, et al. Identification of hub genes and potential CeRNA networks of diabetic cardiomyopathy[J]. Sci Rep, 2023, 13(1): 10258. doi: 10.1038/s41598-023-37378-5
[12] Friedman RC, Farh KK, Burge CB, et al. Most mammalian mRNAs are conserved targets of microRNAs[J]. Genome Res, 2009, 19(1): 92-105. doi: 10.1101/gr.082701.108
[13] Gebert LFR, MacRae IJ. Regulation of microRNA function inanimals[J]. Nat Rev Mol Cell Biol, 2019, 20: 21-37. doi: 10.1038/s41580-018-0045-7
[14] Wang S, Talukder A, Cha M, et al. Computational annotation of miRNA transcription start sites[J]. Brief Bioinform, 2021, 22(1): 380-392. doi: 10.1093/bib/bbz178
[15] Liu HY, Lei C, He Q, et al. Nuclear functions of mammalian microRNAs in gene regulation, immunity and cancer[J]. Mol Cancer, 2018, 17(1): 64. doi: 10.1186/s12943-018-0765-5
[16] Hart M, Walch-Rückheim B, Krammes L, et al. MiR-34a as hub of T cell regulation networks[J]. J Immunother Cancer, 2019, 7(1): 187. doi: 10.1186/s40425-019-0670-5
[17] Sun P, Liu DZ, Jickling GC, et al. MicroRNA-based therapeutics in central nervous system injuries[J]. J Cereb Blood Flow Metab, 2018, 38(7): 1125-1148. doi: 10.1177/0271678X18773871
[18] Khan P, Siddiqui JA, Kshirsagar PG, et al. MicroRNA-1 attenuates the growth and metastasis of small cell lung cancer through CXCR4/FOXM1/RRM2 axis[J]. Mol Cancer, 2023, 22(1): 1. doi: 10.1186/s12943-022-01695-6
[19] Yuan Y, Mei ZT, Qu ZZ, et al. Exosomes secreted from cardiomyocytes suppress the sensitivity of tumor ferroptosis in ischemic heart failure[J]. Signal Transduct Target Ther, 2023, 8(1): 121. doi: 10.1038/s41392-023-01336-4
[20] Sun P, Zhang K, Hassan SH, et al. Endothelium-targeted deletion of microRNA-15a/16-1 promotes poststroke angiogenesis and improves long-term neurological recovery[J]. Circ Res, 2020, 126(8): 1040-1057. doi: 10.1161/CIRCRESAHA.119.315886
[21] Zhang SZ, Tian WC, Duan XX, et al. Melatonin attenuates diabetic cardiomyopathy by increasing autophagy of cardiomyocytes via regulation of VEGF-B/GRP78/PERK signaling pathway[J]. Cardiovasc Diabetol, 2024, 23(1): 19. doi: 10.1186/s12933-023-02078-x
[22] Gou L, Zou H, Li B. Long noncoding RNA MALAT1 knockdown inhibits progression of anaplastic thyroid carcinoma by regulating miR-200a-3p/FOXA1[J]. Cancer Biol Ther, 2019, 20(11): 1355-1365. doi: 10.1080/15384047.2019.1617567
[23] You PH, Chen HC, Han WQ, et al. MiR-200a-3p overexpression alleviates diabetic cardiomyopathy injury in mice by regulating autophagy through the FOXO3/Mst1/Sirt3/AMPK axis[J]. Peer J, 2023, 11: e15840. doi: 10.7717/peerj.15840
[24] Xiao C, Chen MY, Han YP, et al. The protection of luteolin against diabetic cardiomyopathy in rats is related to reversing JNK-suppressed autophagy[J]. Food Funct, 2023, 14(6): 2740-2749. doi: 10.1039/D2FO03871D
[25] Denu RA, Hematti P. Effects of oxidative stress on mesenchymal stem cell biology[J]. Oxid Med Cell Longev, 2016, 2016: 2989076. doi: 10.1155/2016/2989076
[26] Chen GH, Song CC, Pantopoulos K, et al. Mitochondrial oxidative stress mediated Fe-induced ferroptosis via the NRF2-ARE pathway[J]. Free Radic Biol Med, 2022, 180: 95-107. doi: 10.1016/j.freeradbiomed.2022.01.012
[27] Yu ML, Sun YY, Shan XH, et al. Therapeutic overexpression of miR-92a-2-5p ameliorated cardiomyocyte oxidative stress injury in the development of diabetic cardiomyopathy[J]. Cell Mol Biol Lett, 2022, 27(1): 85. doi: 10.1186/s11658-022-00379-9
[28] Zhang WQ, Lu JH, Wang YY, et al. Canagliflozin attenuates lipotoxicity in cardiomyocytes by inhibiting inflammation and ferroptosis through activating AMPK pathway[J]. Int J Mol Sci, 2023, 24(1): 858. doi: 10.3390/ijms24010858
[29] Fang X, Wang H, Han D, et al. Ferroptosis as a target for protection against cardiomyopathy[J]. Proc Natl Acad Sci USA, 2019, 116(7): 2672-2680. doi: 10.1073/pnas.1821022116
[30] Wu F, Shang CX, Jin T, et al. Hispidin inhibits ferroptosis induced by high glucose via the miR-15b-5p/GLS2 axis in pancreatic beta cells[J]. Evid Based Complement Alternat Med, 2023, 2023: 9428241. doi: 10.1155/2023/9428241
[31] Das K, Rao LVM. The role of microRNAs in inflammation[J]. Int J Mol Sci, 2022, 23(24): 15479. doi: 10.3390/ijms232415479
[32] Zhao SM, Tan Y, Qin JN, et al. MicroRNA-223-3p promotes pyroptosis of cardiomyocyte and release of inflammasome factors via downregulating the expression level of SPI1(PU. 1)[J]. Toxicology, 2022, 476: 153252. doi: 10.1016/j.tox.2022.153252
[33] Phang RJ, Ritchie RH, Hausenloy DJ, et al. Cellular interplay between cardiomyocytes and non-myocytes in diabetic cardiomyopathy[J]. Cardiovasc Res, 2023, 119(3): 668-690. doi: 10.1093/cvr/cvac049
[34] Liu GQ, Yan D, Yang L, et al. The effect of miR-471-3p on macrophage polarization in the development of diabetic cardiomyopathy[J]. Life Sci, 2021, 268: 118989. doi: 10.1016/j.lfs.2020.118989
[35] Jia C, Chen H, Wei M, et al. Gold nanoparticle-based miR155 antagonist macrophage delivery restores the cardiac function in ovariectomized diabetic mouse model[J]. Int J Nanomedicine, 2017, 12: 4963-4979. doi: 10.2147/IJN.S138400
[36] Suresh Babu S, Thandavarayan RA, Joladarashi D, et al. MicroRNA-126 overexpression rescues diabetes-induced impairment in efferocytosis of apoptotic cardiomyocytes[J]. Sci Rep, 2016, 6: 36207. doi: 10.1038/srep36207
[37] Zeng C, Wang R, Tan H. Role of pyroptosis in cardiovascular diseases and its therapeutic implications[J]. Int J Biol Sci, 2019, 15(7): 1345-1357. doi: 10.7150/ijbs.33568
[38] Yang L, Cheng CF, Li ZF, et al. Berberine blocks inflammasome activation and alleviates diabetic cardiomyopathy via the miR-18a-3p/Gsdmd pathway[J]. Int J Mol Med, 2023, 51(6): 49. doi: 10.3892/ijmm.2023.5252
[39] Ren L, Chen X, Nie B, et al. Ranolazine inhibits pyroptosis via regulation of miR-135b in the treatment of diabetic cardiac fibrosis[J]. Front Mol Biosci, 2022, 9: 806966. doi: 10.3389/fmolb.2022.806966
[40] Wei J, Zhao Y, Liang H, et al. Preliminary evidence for the presence of multiple forms of cell death in diabetes cardiomyopathy[J]. Acta Pharm Sin B, 2022, 12(1): 1-17. doi: 10.1016/j.apsb.2021.08.026
[41] Cruz-Topete D, He B, Xu XJ, et al. Krüppel-like factor 13 is a major mediator of glucocorticoid receptor signaling in cardiomyocytes and protects these cells from DNA damage and death[J]. J Biol Chem, 2016, 291(37): 19374-19386. doi: 10.1074/jbc.M116.725903
[42] Gu M, Wang J, Wang Y, et al. MiR-147b inhibits cell viability and promotes apoptosis of rat H9c2 cardiomyocytes via down-regulating KLF13 expression[J]. Acta Biochim Biophys Sin(Shanghai), 2018, 50(3): 288-297.
[43] Xu CR, Fang QJ. Inhibiting glucose metabolism by miR-34a and miR-125b protects against Hyperglycemia-induced cardiomyocyte cell death[J]. Arq Bras Cardiol, 2021, 116(3): 415-422. doi: 10.36660/abc.20190529
[44] 王晓燕, 孟建辉, 刘志红, 等. MiR-29a-3p通过靶向Bak1参与高糖诱导的心肌细胞凋亡[J]. 山西医科大学学报, 2023, 54(6): 747-753.
[45] Ma YL, Xu M, Cen XF, et al. Tectorigenin protects against cardiac fibrosis in diabetic mice heart via activating the adiponectin receptor 1-mediated AMPK pathway[J]. Biomedecine Pharmacother, 2024, 174: 116589. doi: 10.1016/j.biopha.2024.116589
[46] Liu BX, Wei Y, He JJ, et al. Human umbilical cord-derived mesenchymal stromal cells improve myocardial fibrosis and restore miRNA-133a expression in diabetic cardiomyopathy[J]. Stem Cell Res Ther, 2024, 15(1): 120. doi: 10.1186/s13287-024-03715-2
-

计量
- 文章访问数: 636
- 施引文献: 0



 下载:
下载: