A case report and meta-analysis for immunoadsorption therapy in patients with dilated cardiomyopathy
-
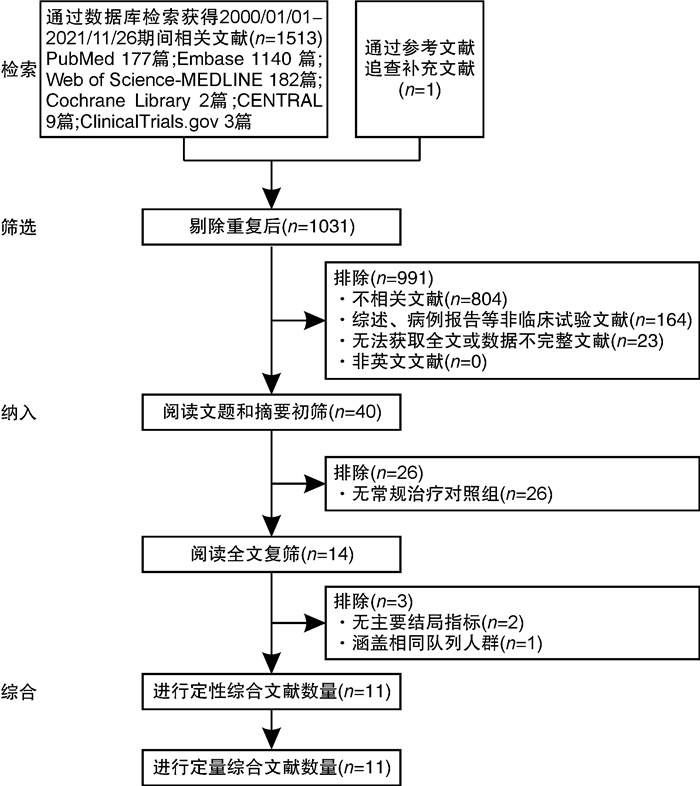
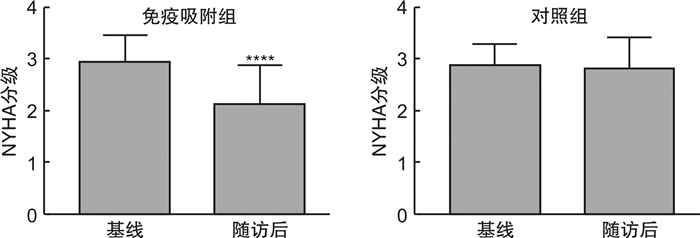
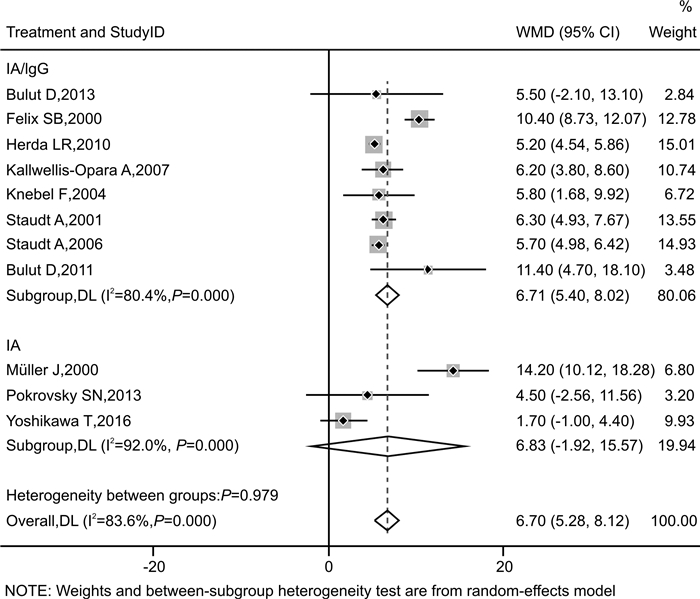
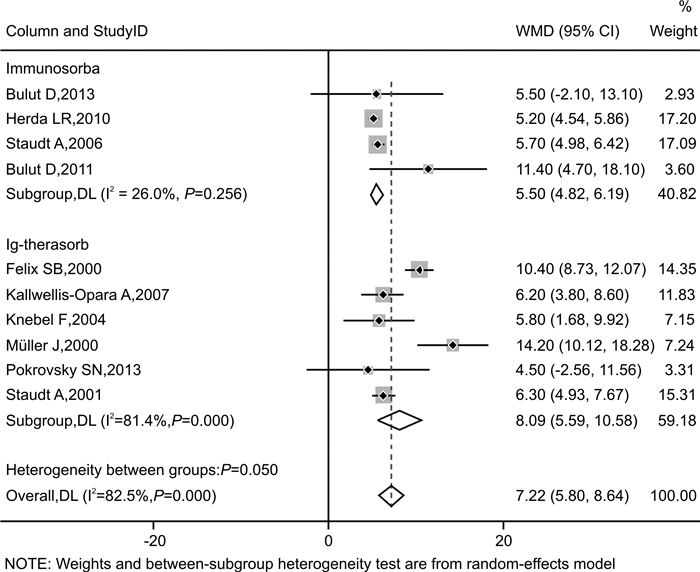
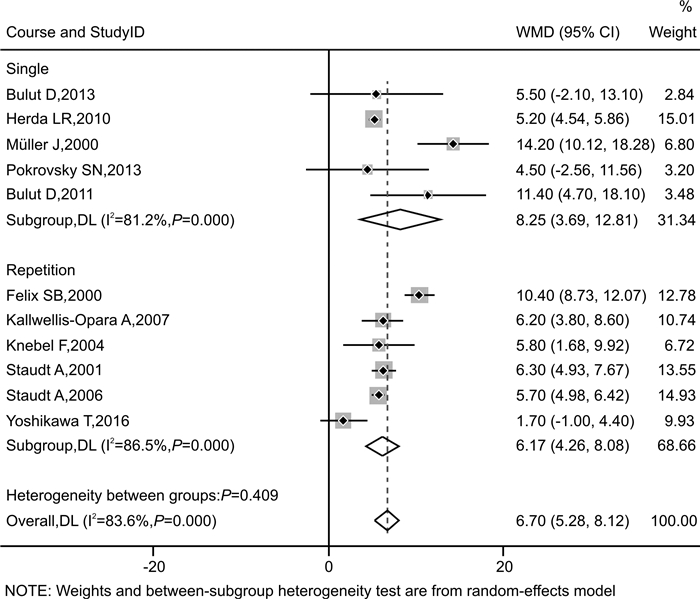
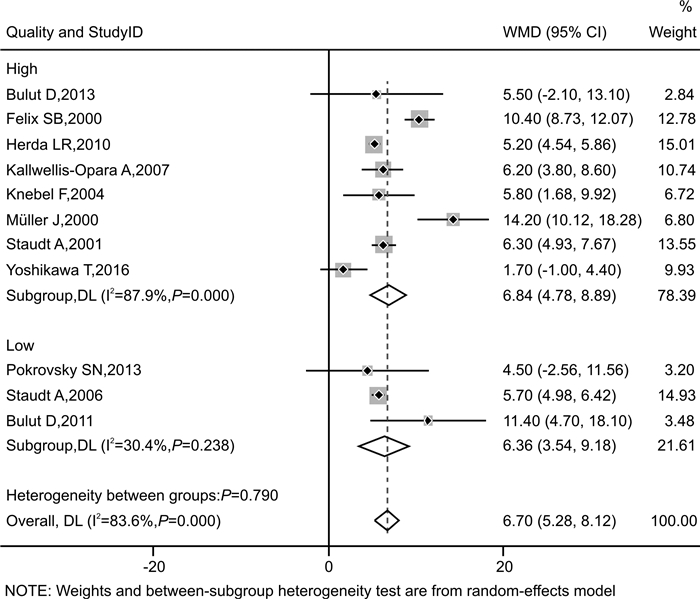
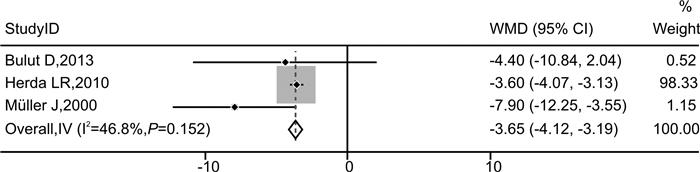
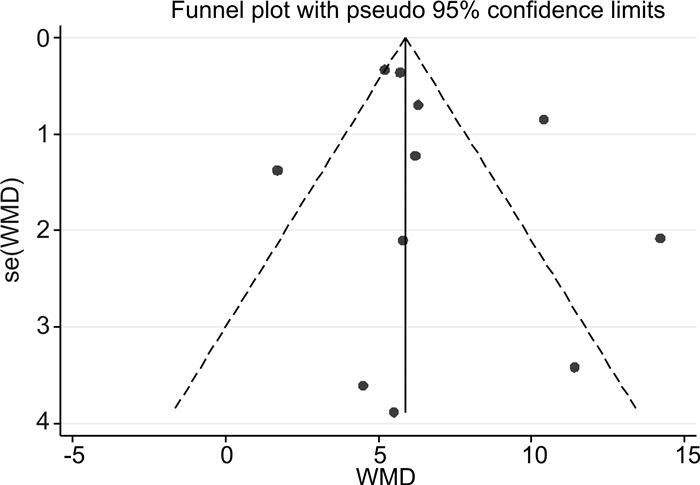
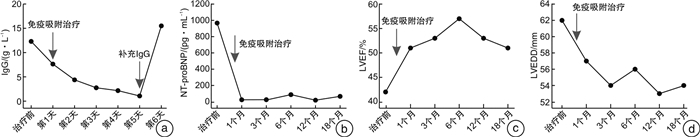
摘要: 目的 探讨免疫吸附疗法应用于扩张型心肌病(DCM)患者的有效性和安全性。方法 本研究在国内首次报道免疫吸附疗法成功应用于1例DCM患者,并通过荟萃分析系统性评价免疫吸附疗法在DCM患者中的有效性与安全性。荟萃分析中,我们检索PubMed、Embase、Web of Science-MEDLINE、Cochrane Library、CENTRAL和ClinicalTrials.gov等数据库,查找2000年1月1日—2021年11月26日以英文发表的免疫吸附疗法治疗DCM的临床研究。应用Stata MP Version 15.0和GraphPad Prism Version 7.0软件进行荟萃分析和统计分析。结果 该例DCM患者接受单疗程的免疫吸附治疗后,在18个月随访期间,N末端脑钠肽原下降,左室射血分数增加以及左室舒张末期内径减低。荟萃分析最终纳入11篇文献,包括313例DCM患者,其中免疫吸附组167例,对照组146例,平均随访时间为3~30个月。与对照组相比,免疫吸附组DCM患者NYHA心功能分级得到明显改善(P < 0.000 1),伴左室射血分数升高(WMD=6.70,95%CI:5.28~8.12)和左室舒张末期内径减低(WMD=-3.65,95%CI:-4.12~-3.19)。免疫吸附组患者未见感染、大出血、肾功能恶化等严重并发症的报道。结论 免疫吸附疗法可明显改善DCM患者的心功能和逆转心脏重构。Abstract: Objective The purpose of this study was to evaluate the efficacy and safety of immunoadsorption (IA) therapy for patients with dilated cardiomyopathy(DCM).Methods For the first in China, we successfully treated one DCM patient with IA therapy. We performed a meta-analysis to systematically evaluate the efficacy and safety of IA therapy in patients with DCM. We searched databases including PubMed, Embase, Web of Science-MEDLINE, Cochrane Library, CENTRAL and ClinicalTrials.gov for clinical trials of IA therapy in DCM patients, which published in English from January 1st, 2000 to November 26th, 2021. Data were analyzed using Stata MP 15.0 and GraphPad Prism 7.0.Results After receiving one course of IA therapy, the DCM patient showed decreased N-terminal pro-B-type natriuretic peptide and left ventricular end-diastolic diameter (LVEDD), and increased left ventricular ejection fraction (LVEF) during the 18 months of follow-up. 11 trials with a total of 313 DCM patients were included in the meta-analysis (n=167 in the IA group and n=146 in the control group). The mean follow-up time ranged from 3 to 30 months. Compared with the control group, DCM patients of the IA group showed improvements in the NYHA functional classification (P < 0.000 1), LVEF (WMD = 6.70, 95%CI: 5.28~8.12) and LVEDD (WMD = -3.65, 95%CI: -4.12~-3.19). In the IA group, no major complications such as infection, major bleeding or worsening of renal function were reported.Conclusion IA therapy significantly improve cardiac function and reverse cardiac remodeling in DCM patients.
-
Key words:
- immunoadsorption /
- dilated cardiomyopathy /
- case report /
- meta-analysis
-

-
表 1 纳入研究的基本特征
Table 1. General data
纳入研究 国家 例数(T/C) 平均年龄/岁(T/C) 平均病程/年(T/C) 干预措施 随访时间 吸附柱 基线LVEF/% 基线NYHA分级 结局指标 T C Pokrovsky SN,2013[7] 俄罗斯 9/7 50.1/49.6 NA IA 常规治疗 6个月 抗IgG柱 ≤35 Ⅱ~Ⅳ ① Bulut D,2013[8] 德国 18/5 50.9/48.7 1.2/1.2 IA/lgG 常规治疗 6个月 蛋白A柱 < 35 Ⅱ~Ⅲ ①②③ Kallwellis-Opara A,2007[9] 德国 6/6 41.5/45.3 NA IA/lgG 常规治疗 3个月 抗IgG柱 < 40 Ⅱ~Ⅲ ① Knebel F,2004[10] 德国 17/17 55.0/57.0 5.8/6.2 IA/lgG 常规治疗 30个月 抗IgG柱 < 35 Ⅱ~Ⅲ ①③ Müller J,2000[11] 德国 17/17 45.9/49.1 3.4/3.5 IA 常规治疗 12个月 抗IgG柱 < 30 Ⅱ~Ⅳ ①②③④ Staudt A,2006[12] 德国 15/15 50.2/52.4 3.6/3.7 IA/lgG 常规治疗 3个月 蛋白A柱 < 35 Ⅲ~Ⅳ ① Felix SB,2000[13] 德国 9/9 49.7/55.3 4.1/4.3 IA/lgG 常规治疗 3个月 抗IgG柱 < 30 Ⅲ~Ⅳ ①③ Staudt A,2001[14] 德国 12/13 50.1/49.8 4.0/3.9 IA/lgG 常规治疗 3个月 抗IgG柱 < 30 Ⅲ~Ⅳ ① Herda LR,2010[15] 德国 30/30 54.7/52.6 2.1/2.9 IA/lgG 常规治疗 3个月 蛋白A柱 ≤45 Ⅱ~Ⅳ ①② Yoshikawa T,2016[16] 日本 21/22 56.0/56.0 9.6/6.1 IA *延迟IA治疗 12个月 色氨酸柱 < 35 Ⅲ~Ⅳ ①③④ Bulut D,2011[17] 德国 13/5 52.3/49.8 NA IA/lgG 常规治疗 6个月 蛋白A柱 < 35 Ⅲ~Ⅳ ① 注:T:IA组;C:对照组;NA:无法获取;抗IgG柱:即羊抗人IgG吸附柱;①基线与随访后两组LVEF变化情况;②基线与随访后两组LVEDD变化情况;③基线与随访后两组NYHA心功能分级变化情况;④安全性事件。*延迟3个月进行IA治疗;试验开始后的3个月未进行IA治疗,在这段时间里将其视为常规治疗对照组。 表 2 随机对照试验的改良JADAD量表评估结果
Table 2. Evaluation results of the modified JADAD scale in randomized controlled trials
表 3 队列研究的Newcastle-Ottawa Scale量表评估结果
Table 3. Newcastle-Ottawa Scale assessment results of cohort studies
研究 选择 组间可比性 结局 得分 暴露组的代表性 非暴露组的代表性 暴露因素确定 研究起始时结局指标的确定性 结局指标的评价 为观察到结局发生,随访是否充分(6个月) 随访的完整性 Bulut D,2013[8] ☆ ☆ ★ ★ ★☆ ★ ★ ★ 6 Kallwellis-Opara A,2007[9] ☆ ☆ ★ ★ ★★ ★ ☆ ★ 6 Knebel F,2004[10] ☆ ☆ ★ ☆ ★★ ★ ★ ★ 6 Müller J,2000[11] ★ ★ ★ ★ ★★ ★ ★ ★ 9 Staudt A,2006[12] ☆ ☆ ★ ☆ ★★ ★ ☆ ★ 5 Herda LR,2010[15] ★ ★ ★ ★ ★★ ★ ★ ★ 9 Bulut D,2011[17] ☆ ☆ ★ ★ ★☆ ★ ☆ ★ 5 注:IA治疗后约在6~12个月达最佳疗效[28];因此在定义随访是否充分时,选择6个月为界限值。 表 4 LVEF研究的敏感性分析结果
Table 4. Sensitivity analysis results of the LVEF study
排除研究 异质性 合并统计量WMD及95%CI Pokrovsky SN, 2013[7] I2=82.5% P=0.000 6.78(5.33,8.23) Bulut D, 2013[8] I2=85.2% P=0.001 6.74(5.29,8.20) Kallwellis-Opara A,2007[9] I2=85.2% P=0.000 6.78(5.23,9.87) Knebel F,2004[10] I2=85.2% P=0.000 6.78(5.28,8.27) Müller J,2000[11] I2=79.9% P=0.000 6.16(4.86,7.46) Staudt A,2006[12] I2=85.2% P=0.000 6.96(5.03,8.89) Felix SB,2000[13] I2=70.8% P=0.000 5.99(4.81,7.17) Staudt A,2001[14] I2=85.1% P=0.000 6.80(5.16,8.43) Herda LR,2010[15] I2=83.4% P=0.000 7.03(5.17,8.89) Yoshikawa T,2016[16] I2=82.5% P=0.000 7.22(5.80,8.64) Bulut D,2011[17] I2=84.6% P=0.000 6.53(5.10,7.96) Müller J,2000[11] I2=0.0% P=0.576 5.57(5.13,6.02) Felix SB, 2000[13] Yoshikawa T, 2016[16] -
[1] 中华医学会心血管病学分会, 中国心肌炎心肌病协作组. 中国扩张型心肌病诊断和治疗指南[J]. 临床心血管病杂志, 2018, 34(05): 421-434. https://www.cnki.com.cn/Article/CJFDTOTAL-LCXB201805001.htm
[2] Cooper LT Jr. The natural history and role of immunoadsorption in dilated cardiomyopathy[J]. J Clin Apher, 2005, 20(4): 256-260. doi: 10.1002/jca.20045
[3] Ikeda U, Kasai H, Izawa A, et al. Immunoadsorption therapy for patients with dilated cardiomyopathy and heart failure[J]. Curr Cardiol Rev, 2008, 4(3): 219-222. doi: 10.2174/157340308785160534
[4] Caforio AL, Tona F, Bottaro S, et al. Clinical implications of anti-heart autoantibodies in myocarditis and dilated cardiomyopathy[J]. Autoimmunity, 2008, 41(1): 35-45. doi: 10.1080/08916930701619235
[5] Dandel M, Wallukat G, Potapov E, et al. Role of β1-adrenoceptor autoantibodies in the pathogenesis of dilated cardiomyopathy[J]. Immunobiology, 2012, 217(5): 511-520. doi: 10.1016/j.imbio.2011.07.012
[6] Wallukat G, Reinke P, Dörffel WV, et al. Removal of autoantibodies in dilated cardiomyopathy by immunoadsorption[J]. Int J Cardiol, 1996, 54(2): 191-195. doi: 10.1016/0167-5273(96)02598-3
[7] Pokrovsky SN, Ezhov MV, Safarova MS, et al. Ig apheresis for the treatment of severe DCM patients[J]. Atheroscler Suppl, 2013, 14(1): 213-218. doi: 10.1016/j.atherosclerosissup.2012.10.028
[8] Bulut D, Creutzenberg G, Mügge A. The number of regulatory T cells correlates with hemodynamic improvement in patients with inflammatory dilated cardiomyopathy after immunoadsorption therapy[J]. Scand J Immunol, 2013, 77(1): 54-61. doi: 10.1111/sji.12000
[9] Kallwellis-Opara A, Staudt A, Trimpert C, et al. Immunoadsorption and subsequent immunoglobulin substitution decreases myocardial gene expression of desmin in dilated cardiomyopathy[J]. J Mol Med(Berl), 2007, 85(12): 1429-1435.
[10] Knebel F, Böhm M, Staudt A, et al. Reduction of morbidity by immunoadsorption therapy in patients with dilated cardiomyopathy[J]. Int J Cardiol, 2004, 97(3): 517-520. doi: 10.1016/j.ijcard.2003.12.003
[11] Müller J, Wallukat G, Dandel M, et al. Immunoglobulin adsorption in patients with idiopathic dilated cardiomyopathy[J]. Circulation, 2000, 101(4): 385-391. doi: 10.1161/01.CIR.101.4.385
[12] Staudt A, Staudt Y, Hummel A, et al. Effects of Immunoadsorption on the nt-BNP and nt-ANP Plasma Levels of Patients Suffering From Dilated Cardiomyopathy[J]. Ther Apher Dial, 2006, 10(1): 42-48. doi: 10.1111/j.1744-9987.2006.00343.x
[13] Felix SB, Staudt A, Dörffel WV, et al. Hemodynamic effects of immunoadsorption and subsequent immunoglobulin substitution in dilated cardiomyopathy: three-month results from a randomized study[J]. J Am Coll Cardiol, 2000, 35(6): 1590-1598. doi: 10.1016/S0735-1097(00)00568-4
[14] Staudt A, Schäper F, Stangl V, et al. Immunohistological changes in dilated cardiomyopathy induced by immunoadsorption therapy and subsequent immunoglobulin substitution[J]. Circulation, 2001, 103(22): 2681-2686. doi: 10.1161/01.CIR.103.22.2681
[15] Herda LR, Trimpert C, Nauke U, et al. Effects of immunoadsorption and subsequent immunoglobulin G substitution on cardiopulmonary exercise capacity in patients with dilated cardiomyopathy[J]. Am Heart J, 2010, 159(5): 809-816. doi: 10.1016/j.ahj.2010.01.012
[16] Yoshikawa T, Baba A, Akaishi M, et al. Immunoadsorption therapy for dilated cardiomyopathy using tryptophan columnA prospective, multicenter, randomized, within-patient and parallel-group comparative study to evaluate efficacy and safety[J]. J Clin Apher, 2016, 31(6): 535-544. doi: 10.1002/jca.21446
[17] Bulut D, Scheeler M, Niedballa LM, et al. Effects of immunoadsorption on endothelial function, circulating endothelial progenitor cells and circulating microparticles in patients with inflammatory dilated cardiomyopathy[J]. Clin Res Cardiol, 2011, 100(7): 603-610. doi: 10.1007/s00392-011-0287-2
[18] Bian RT, Wang ZT, Li WY. Immunoadsorption treatment for dilated cardiomyopathy: A PRISMA-compliant systematic review and meta-analysis[J]. Medicine(Baltimore), 2021, 100(26): e26475.
[19] Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary?[J]. Control Clin Trials, 1996, 17(1): 1-12. doi: 10.1016/0197-2456(95)00134-4
[20] Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa scale(NOS)for assessing the quality of nonrandomised studies in meta-analyses[J]. Ottawa: Ottawa Hospital Research Institute, 2011: 1-12.
[21] Schwartz J, Padmanabhan A, Aqui N, et al. Guidelines on the Use of Therapeutic Apheresis in Clinical Practice-Evidence-Based Approach from the Writing Committee of the American Society for Apheresis: The Seventh Special Issue[J]. J Clin Apher, 2016, 31(3): 149-162.
[22] Staudt A, Hummel A, Ruppert J, et al. Immunoadsorption in dilated cardiomyopathy: 6-month results from a randomized study[J]. Am Heart J, 2006, 152(4): 712. e711-716. doi: 10.1016/j.ahj.2006.06.027
[23] Reinthaler M, Empen K, Herda LR, et al. The effect of a repeated immunoadsorption in patients with dilated cardiomyopathy after recurrence of severe heart failure symptoms[J]. J Clin Apher, 2015, 30(4): 217-223.
[24] Doesch AO, Konstandin M, Celik S, et al. Effects of protein A immunoadsorption in patients with advanced chronic dilated cardiomyopathy[J]. J Clin Apher, 2009, 24(4): 141-149.
[25] Dwyer JM. Manipulating the immune system with immune globulin[J]. N Engl J Med, 1992, 326(2): 107-116. doi: 10.1056/NEJM199201093260206
[26] Roifman CM, Levison H, Gelfand EW. High-dose versus low-dose intravenous immunoglobulin in hypogammaglobulinaemia and chronic lung disease[J]. Lancet, 1987, 1(8541): 1075-1077.
[27] Staudt A, Dörr M, Staudt Y, et al. Role of immunoglobulin G3 subclass in dilated cardiomyopathy: results from protein A immunoadsorption[J]. Am Heart J, 2005, 150(4): 729-736. doi: 10.1016/j.ahj.2004.11.002
[28] Dandel M, Wallukat G, Englert A, et al. Long-term benefits of immunoadsorption in β(1)-adrenoceptor autoantibody-positive transplant candidates with dilated cardiomyopathy[J]. Eur J Heart Fail, 2012, 14(12): 1374-1388. doi: 10.1093/eurjhf/hfs123
-




 下载:
下载: