The steady-state time of platelet inhibition by clopidogrel detected by light transmission aggregation
-
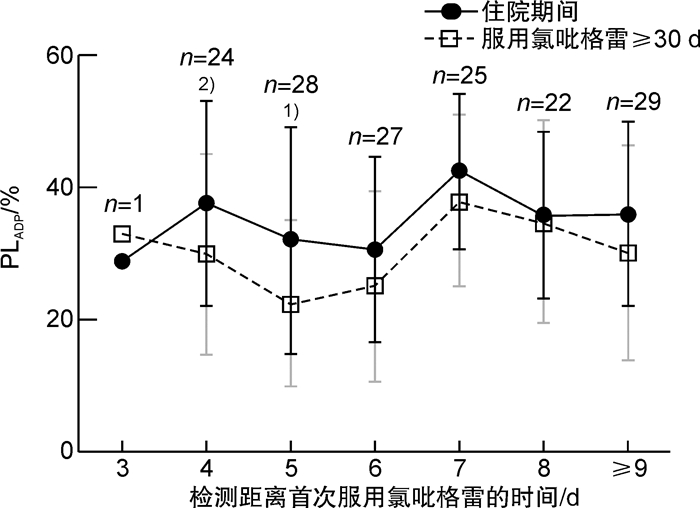
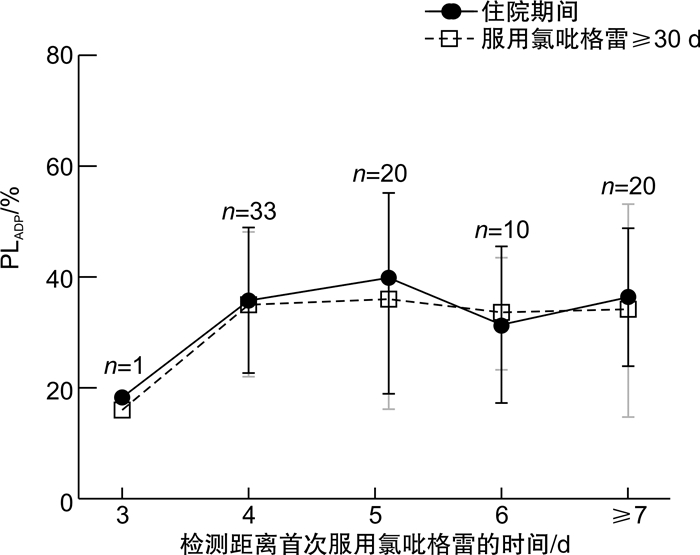
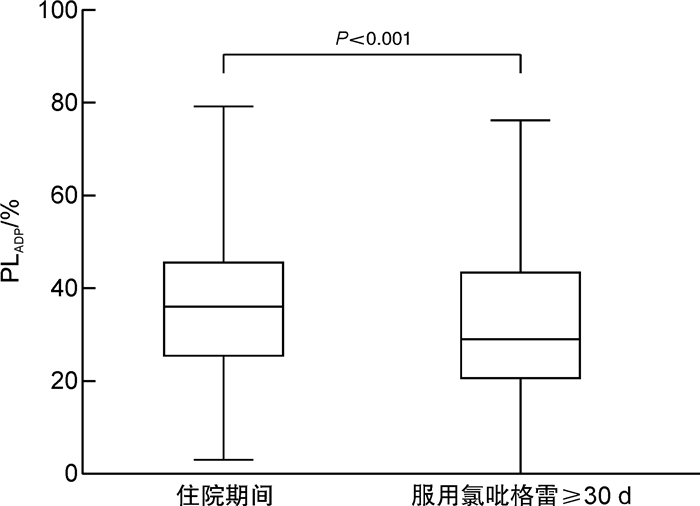
摘要: 目的 探究冠心病患者服用氯吡格雷后血小板抑制作用达到稳态的时间,为氯吡格雷药效学研究提供借鉴。方法 入选接受经皮冠状动脉介入治疗且服用氯吡格雷3 d后的冠心病患者,采用光比浊法检测二磷酸腺苷诱导的血小板聚集率(PLADP)。分别比较检测时已服用氯吡格雷不同天数患者住院期间和服用氯吡格雷30 d后的PLADP水平。结果 入选240例患者,住院期间及出院后检测PLADP距离首次服用氯吡格雷的时间分别为6(5,8) d和39(35,47) d,患者住院期间PLADP水平显著高于服用氯吡格雷≥30 d的PLADP水平[36(25,46)% vs 29(20,44)%,P< 0.01]。入选患者中未服用负荷剂量(仅予维持剂量75 mg/d)氯吡格雷者156例,住院期间PLADP水平显著高于服用氯吡格雷≥30 d PLADP水平[36(25,46)% vs 28(19,42)%,P< 0.01];此类患者中住院期间服用氯吡格雷≥6 d者,住院期间与服用氯吡格雷30 d的PLADP水平差异无统计学意义(P>0.05)。入选患者中服用氯吡格雷负荷剂量(150 mg或300 mg)者84例,住院期间与服用氯吡格雷≥30 d的PLADP水平差异无统计学意义[(35.99±15.38)% vs (32.61±16.30)%,P>0.05];检测时已服用氯吡格雷不同天数患者住院期间与服用氯吡格雷30 d的PLADP水平差异均无统计学意义(P>0.05)。结论 冠心病患者服用维持量氯吡格雷≥6 d或负荷加维持量氯吡格雷≥3 d时残余血小板聚集功能与服用氯吡格雷30 d后相当;在以上时间窗检测的PLADP水平可准确体现氯吡格雷的长期抗血小板疗效。Abstract: Objective To explore the steady-state time of platelet inhibition by clopidogrel and provide a reference for the pharmacodynamic study of clopidogrel.Methods Patients with coronary artery disease undergoing percutaneous coronary intervention(PCI) were recruited after their taking clopidogrel for more than 3 days. Platelet aggregation induced by adenosine diphosphate(PLADP) was measured by light transmission aggregation(LTA). PLADP levels of patients who had taken clopidogrel for different days at the time of detection were compared during hospitalization and after clopidogrel administration ≥30 days.Results A total of 240 patients were enrolled in the study. The time between detection of platelet aggregation function and initial administration of clopidogrel was 6(5, 8) and 39(35, 47) days in hospitalization and after discharge, respectively. Patients'PLADP levels measured during the hospital were significantly higher than those measured after clopidogrel administration ≥30 days[36(25, 46)% vs 29(20, 44)%,P< 0.01]. For the 156 patients who were treated without loading-dose(only a maintenance dose of 75 mg/day) clopidogrel, a similar result was found. i. e. the average in-hospital PLADP level was significantly higher than that measured after clopidogrel administration ≥30 days[36(25, 46)% vs 28(19, 42)%,P< 0.01]. However, no significant difference was found for each group of patients in whom the detection time point exceeded 6 days of clopidogrel treatment(P>0.05). In addition, for the 84 patients who were treated with loading-dose clopidogrel of either 150 mg or 300 mg daily, no significant difference in PLADP level was found comparing the in-hospital levels and that measured after clopidogrel administration ≥30 days[(35.99±15.38)% vs (32.61±16.30)%,P>0.05]. There was no significant difference in PLADP for patients who had taken clopidogrel for different days during hospitalization and after clopidogrel administration ≥30 days(P>0.05).Conclusion The in-hospital residual platelet aggregation would be equivalent to that measured after clopidogrel administration ≥30 days if a patient takes a maintenance-dose clopidogrel for ≥6 days or loading-plus maintenance-dose clopidogrel for ≥3 days. The PLADP levels that meet the above detection window would be accurately reflect the long-term pharmacodynamic effect of clopidogrel.
-
Key words:
- coronary artery disease /
- clopidogrel /
- platelet aggregation /
- pharmacodynamics
-

-
-
[1] Kamran H, Jneid H, Kayani WT, et al. Oral Antiplatelet Therapy After Acute Coronary Syndrome: A Review[J]. JAMA, 2021, 325(15): 1545-1555. doi: 10.1001/jama.2021.0716
[2] 易加祎, 郑昕. 急性ST段抬高型心肌梗死溶栓患者的抗血小板治疗进展[J]. 临床心血管病杂志, 2020, 36(6): 502-505. https://www.cnki.com.cn/Article/CJFDTOTAL-LCXB202006003.htm
[3] Stuckey TD, Kirtane AJ, Brodie BR, et al. Impact of Aspirin and Clopidogrel Hyporesponsiveness in Patients Treated With Drug-Eluting Stents: 2-Year Results of a Prospective, Multicenter Registry Study[J]. JACC Cardiovasc Interv, 2017, 10(16): 1607-1617. doi: 10.1016/j.jcin.2017.05.059
[4] Al-Husein BA, Al-Azzam SI, Alzoubi KH, et al. Investigating the Effect of Demographics, Clinical Characteristics, and Polymorphism of MDR-1, CYP1A2, CYP3A4, and CYP3A5 on Clopidogrel Resistance[J]. J Cardiovasc Pharmacol, 2018, 72(6): 296-302. doi: 10.1097/FJC.0000000000000627
[5] Gao XF, Lu S, Ge Z, et al. Relationship between high platelet reactivity on clopidogrel and long-term clinical outcomes after drug-eluting stents implantation(PAINT-DES): a prospective, propensity score-matched cohort study[J]. BMC Cardiovasc Disord, 2018, 18(1): 103. doi: 10.1186/s12872-018-0841-1
[6] Collet JP, Thiele H, Barbato E, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation[J]. Eur Heart J, 2020, 42(14): 1289-1367.
[7] Galli M, Benenati S, Capodanno D, et al. Guided versus standard antiplatelet therapy in patients undergoing percutaneous coronary intervention: a systematic review and meta-analysis[J]. Lancet, 2021, 397(10283): 1470-1483. doi: 10.1016/S0140-6736(21)00533-X
[8] Sun P, McMillan-Ward E, Mian R, et al. Comparison of light transmission aggregometry and multiple electrode aggregometry for the evaluation of patients with mucocutaneous bleeding[J]. Int J Lab Hematol, 2019, 41(1): 133-140. doi: 10.1111/ijlh.12937
[9] Varvat J, Montmartin A, Epinat M, et al. Monitoring of biological response to clopidogrel after treatment for non-cardioembolic ischemic stroke or transient ischemic attack[J]. Am J Transl Res, 2019, 11(9): 5332-5337.
[10] 世界华人检验与病理医师协会, 中国医师协会检验医师分会心血管检验医学专业委员会. 血小板功能检测在急性冠脉综合征患者抗血小板治疗中的应用专家共识[J]. 中华医学杂志, 2018, 98(22): 1743-1751. doi: 10.3760/cma.j.issn.0376-2491.2018.22.005
[11] Chouchene S, Dabboubi R, Raddaoui H, et al. Clopidogrel utilization in patients with coronary artery disease and diabetes mellitus: should we determine CYP2C19*2 genotype?[J]. Eur J Clin Pharmacol, 2018, 74(12): 1567-1574. doi: 10.1007/s00228-018-2530-5
[12] Xu K, Ye S, Zhang S, et al. Impact of Platelet Endothelial Aggregation Receptor-1 Genotypes on Platelet Reactivity and Early Cardiovascular Outcomes in Patients Undergoing Percutaneous Coronary Intervention and Treated With Aspirin and Clopidogrel[J]. Circ Cardiovasc Interv, 2019, 12(5): e007019. doi: 10.1161/CIRCINTERVENTIONS.118.007019
[13] Patti G, Cavallari I, Andreotti F, et al. Prevention of atherothrombotic events in patients with diabetes mellitus: from antithrombotic therapies to new-generation glucose-lowering drugs[J]. Nat Rev Cardiol, 2019, 16(2): 113-130. doi: 10.1038/s41569-018-0080-2
[14] Jiang Z, Zhang R, Sun M, et al. Effect of Clopidogrel vs Ticagrelor on Platelet Aggregation and Inflammation Markers After Percutaneous Coronary Intervention for ST-Elevation Myocardial Infarction[J]. Can J Cardiol, 2018, 34(12): 1606-1612. doi: 10.1016/j.cjca.2018.08.024
[15] Wang J, Abdus S, Tan C, et al. Serum uric acid level negatively correlated with the prevalence of clopidogrel low response in patients undergoing antiplatelet treatment with aspirin and clopidogrel[J]. Nutr Metab Cardiovasc Dis, 2020, 30(12): 2215-2220. doi: 10.1016/j.numecd.2020.07.025
[16] Zhao X, Li Q, Tu C, et al. High glycated albumin is an independent predictor of low response to clopidogrel in ACS patients: a cross-sectional study[J]. Cardiovasc Diabetol, 2020, 19(1): 171. doi: 10.1186/s12933-020-01146-w
[17] Le Blanc J, Mullier F, Vayne C, et al. Advances in Platelet Function Testing-Light Transmission Aggregometry and Beyond[J]. J Clin Med, 2020, 9(8).
[18] Zhang YZ, Chen BL, Zhang W, et al. Non-antiplatelet effect of clopidogrel: improving endothelial function in Chinese healthy subjects with different CYP2C19 genotype[J]. Clin Exp Pharmacol Physiol, 2015, 42(1): 22-26. doi: 10.1111/1440-1681.12325
[19] 舒雪梅, 郭涛. 血小板受体作为抗血小板治疗靶点的研究进展[J]. 临床血液学杂志, 2020, 33(1): 13-17. https://www.cnki.com.cn/Article/CJFDTOTAL-LCXZ202001005.htm
[20] Zhang M, Wang J, Zhang Y, et al. Impacts of CYP2C19 Polymorphism and Clopidogrel Dosing on in-Stent Restenosis: A Retrospective Cohort Study in Chinese Patients[J]. Drug Des Devel Ther, 2020, 14: 669-676. doi: 10.2147/DDDT.S242167
[21] Martínez-Quintana E, Medina-Gil JM, Rodríguez-González F, et al. Positive clinical response to clopidogrel is independent of paraoxonase 1 Q192R and CYP2C19 genetic variants[J]. J Clin Pharmacol, 2014, 54(8): 843-849. doi: 10.1002/jcph.275
[22] Pereira NL, Rihal CS, So D, et al. Clopidogrel Pharmacogenetics[J]. Circ Cardiovasc Interv, 2019, 12(4): e007811. doi: 10.1161/CIRCINTERVENTIONS.119.007811
[23] Wang W, Shao C, Xu B, et al. CYP2C19 genotype has prognostic value in specific populations following coronary stenting[J]. Ann Transl Med, 2021, 9(13): 1066. doi: 10.21037/atm-20-7724
[24] Price MJ, Berger PB, Teirstein PS, et al. Standard-vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial[J]. JAMA, 2011, 305(11): 1097-105. doi: 10.1001/jama.2011.290
[25] Karazniewicz-Łada M, Danielak D, Burchardt P, et al. Clinical pharmacokinetics of clopidogrel and its metabolites in patients with cardiovascular diseases[J]. Clin Pharmacokinet, 2014, 53(2): 155-164. doi: 10.1007/s40262-013-0105-2
[26] Horenstein RB, Madabushi R, Zineh I, et al. Effectiveness of clopidogrel dose escalation to normalize active metabolite exposure and antiplatelet effects in CYP2C19 poor metabolizers[J]. J Clin Pharmacol, 2014, 54(8): 865-873. doi: 10.1002/jcph.293
-




 下载:
下载: