The improved exercise capacity of pulmonary arterial hypertension-specific drug therapy for Eisenmenger syndrome and related factors: A meta-analysis
-
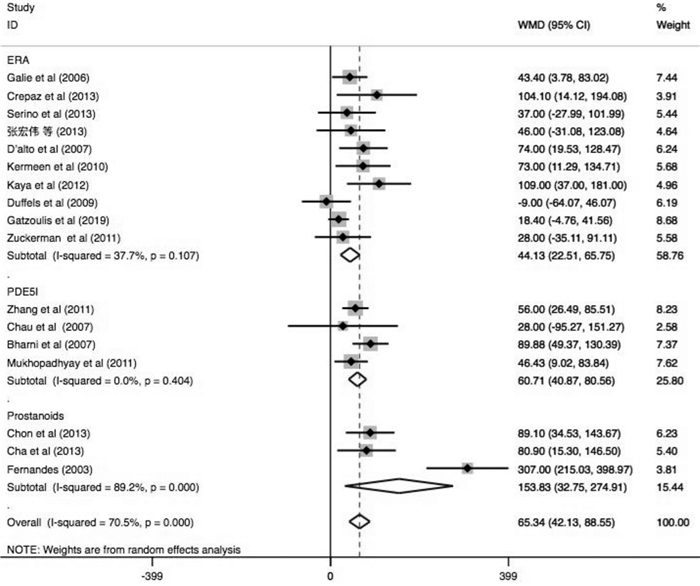
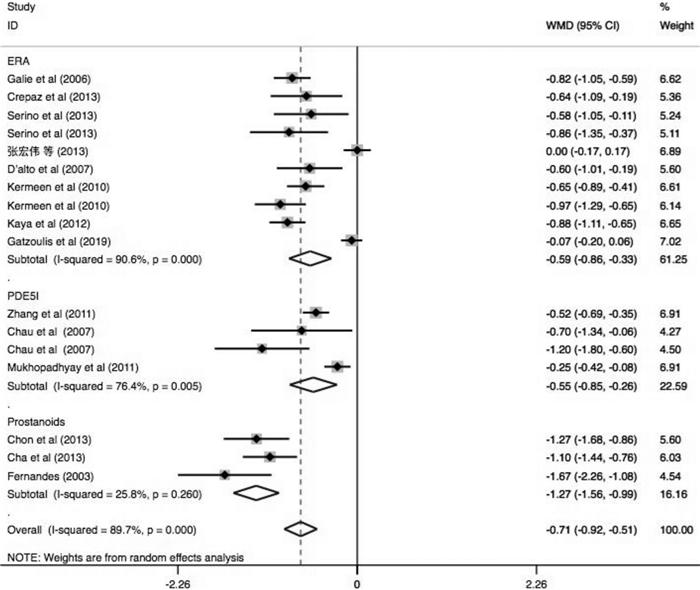
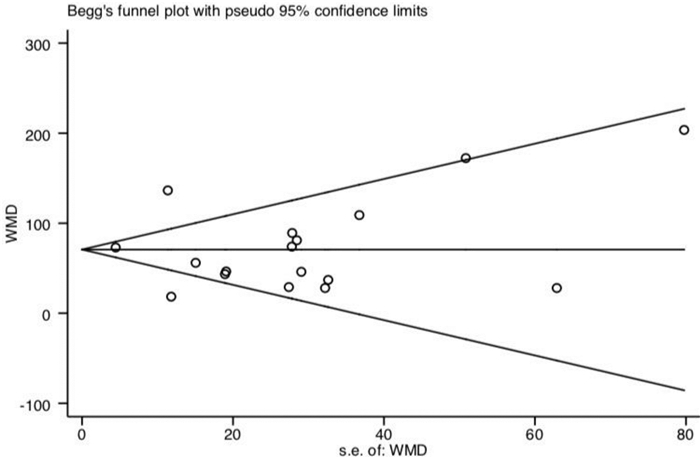
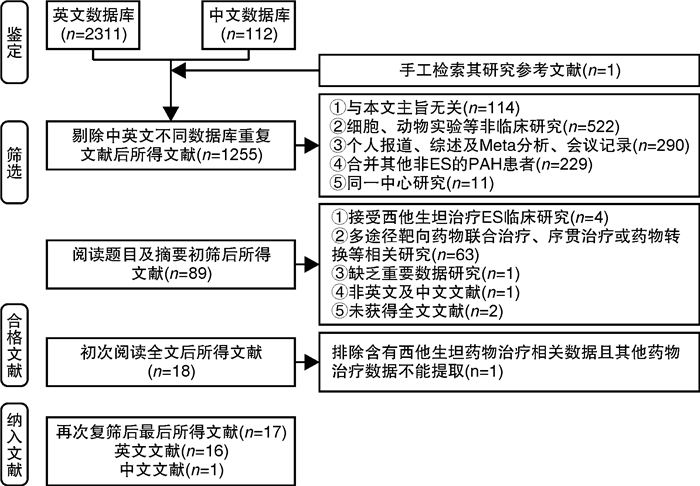
摘要: 目的 系统评价肺动脉高压(PAH)靶向药物改善艾森门格综合征(ES)的运动耐量及相关因素分析。方法 由2名研究员对中文数据库及英文数据库进行独立检索,分别依据纳入及排除标准进行文献筛选、数据提取及质量评价。依据异质性检验结果决定采用效应模型,采用Stata 14.1统计软件进行分析,其中连续性资料采用加权均数差(WMD)及95%置信区间(CI)表示。结果 17篇文献共纳入485例ES患者,包括内皮素受体拮抗剂(ERA)研究10项,磷酸二酯酶5抑制剂(PDE5i)4项及前列环素类3项,治疗期间仅2.6%患者出现死亡,临床事件恶化率不足10%。研究结果显示靶向药物可有效改善ES患者的运动耐量,进一步分析则发现其疗效差异与靶向药物类型、ES患者人群平均年龄、药物治疗时间及是否合并唐氏综合征(DS)存在关系。ERA可提高20~30岁人群6 min步行距离(6MWD)约104.1 m(95%CI:14.12~194.08,P=0.023)及30~40岁人群6MWD约40.88 m(95%CI:17.72~64.04,P=0.001);PDE5i类药物虽可明显改善20~30岁人群的运动耐量(6MWD:WMD=+62.16 m,P<0.0001),但对30~40岁人群的运动耐量改善并不明显(6MWD:WMD=+28.00 m,P=0.656);前列环素类药物则对于30岁以上患者的6MWD改善明显(30~40岁:WMD=+307 m;>40岁:WMD=+85.75 m)。短期药物治疗(12个月以内)可明显增加纳入ES患者的6MWD约58.56 m(P<0.0001),同时改善临床心功能水平(WMD=-0.68,P<0.0001),持续靶向药物治疗则可进一步增加该类患者的运动耐量(P<0.0001)。短期口服波生坦虽不能有效提高ES合并DS患者的6MWD(WMD=+35.50 m,95%CI:-5.89~76.88,P=0.093;I2=0.0%),但长期药物治疗后可明显改善其运动耐量(P=0.005)。结论 早期靶向药物治疗ES可明显改善患者运动耐量,同时需根据患者的临床特征选择较好的药物方案及治疗时间。Abstract: Objective To evaluate changes and related factors of exercise tolerance in pulmonary arterial hypertension(PAH) -specific drugs for Eisenmenger Syndrome(ES) systematically.Methods Two investigators have searched Chinese databases and English databases, independently. After that, literature screening, data extraction and quality assessment were performed. The analyses used Stata 14.1 where weighted mean difference(WMD) with 95% confidence interval(CI) were calculated for the continuous data. And a randomized-effect model or a fixed-effect model was applied according to the results of the heterogeneity test.Results Seventeen studies those met the inclusion criteria were enrolled in this review, involving485 patients with ES. It included studies about endothelin receptor antagonists(ERAs,n=10), phosophodiesterase type 5 inhibitors(PDE5i,n=4) and prostanoids(n=3). Only 2.6% of patients died, and the rate of clinical worsening was not reach on 10%. Outcomes have showed that specific drugs could effectively improve exercise tolerance in ES patients, and it further indicated the differences might be resulted from: 1) type of the targeted drugs; 2) mean age of ES patient group; 3) The duration of therapeutic administration and 4) Down's syndrome. We found that ERAs could increase 6MWD by 104.1 meters(95%CI: 14.12-194.08,P=0.023) in groups aged 20-30 and 40.88 meters(95%CI: 17.72-64.04,P=0.001) in groups aged 30-40. Although PDE5i drugs could significantly improve exercise capacity of individuals of a mean age at 20-30 years old level(6MWD:WMD=+62.16 meters,P< 0.0001), but not significantly improve that of ES patients at 30-40 year-old level(6MWD:WMD=+ 28.00 meters,P=0.656). Prostacyclin drugs greatly improved 6MWD in patients over 30 years old(30-40 years old:WMD=+307 meters; >40 years old:WMD=+85.75 meters). Short-term drug therapy(< 12 months) significantly increased the 6MWD of ES patients by 58.56 meters(P< 0.0001) and improved clinical cardiac function(WMD=-0.68,P< 0.0001), while continued targeted drug therapy further increased exercise tolerance of these patients(P< 0.0001). Although a short-term bosentan could not effectively improve the 6MWD of ES patients with DS(WMD=+35.50 meters, 95%CI: -5.89-76.88,P=0.093;I2=0.0%), but a long-term treatment could significantly improve exercise tolerance(P=0.005).Conclusion Early PAH-specific drugs should be applied for ES to improve exercise tolerances significantly. And the detailed therapeutic regimens and duration can be selected based on the clinical characteristics of ES patients for a better prognosis.
-

-
表 1 文献检索策略
Table 1. Search strategy
中文 英文 #1 内皮素受体拮抗剂OR波生坦OR安利生坦OR马西替坦 #1 endothelin receptor antagonist OR ERA OR bosentan OR ambrisentan OR macitentan #2 磷酸二酯酶5抑制剂OR西地那非OR他达那非 #2 phosphodiesterase type 5 inhibitor OR PDE5i OR sildenafil OR tadalafil #3 前列环素类似物OR伊洛前列素OR贝前列素OR曲前列环素OR依前列醇 #3 prostanoids OR prostacyclin analogs OR iloprost OR beraprost OR treprostinil OR epoprostenol #4 艾森门格OR艾森曼格OR Eisenmenger syndrome OR ES #4 Eisenmenger syndrome OR ES #5 #1 OR #2 OR#3 #5 #1 OR #2 OR#3 #6 #4 AND#5 #6 #4 AND#5 表 2 纳入文献的基本特征
Table 2. Basic characteristics of enrolled studies
纳入研究 时间 设计 地区 人数/例 药物 年龄/岁 病因分类 DS(是/否) 时间/月 心功能分级 主要结局 ERAs Gali等[9] 2006 RCT 意大利 37 波生坦 37.2(12.0) ASD(21.6%)
VSD(64.9%)
Com(13.5%)否 4 NA 6MWD Crepaz等[10] 2013 NRCT 意大利 7 波生坦 29.6(11.2) VSD(100.0%) 是(100%) 24 3.0(-) 6MWD Serino等[11] 2013 NRCT 意大利 7 波生坦 31.7(-) VSD(71.4%)
Com(28.6%)是(100%) 24 3.3(0.5) 6MWD 张宏伟等[12] 2013 NRCT 中国 22 波生坦 10.0(5.0) ASD(4.5%)
VSD(50.0%)
PDA(13.6%)
Com(31.9%)否 7 2.1(0.3) 6MWD D’alto等[13] 2007 NRCT 意大利 22 波生坦 38.0(10.0) ASD(4.5%)
VSD(54.5%)
Com(41.0%)否 12 3.1(0.7) 6MWD Kermeen等[14] 2010 NRCT 新西兰 53 波生坦 34.0(12.0) ASD(3.8%)
VSD(37.7%)
PDA(1.9%)
Com(45.3%)是(32.1%) 24 3.2(0.5) 6MWD Kaya等[15] 2012 NRCT 土耳其 23 波生坦 31.0(12.0) ASD(26.1%)
VSD(65.2%)
PDA(8.7%)否 24 3.2(0.4) 6MWD Duffels等[16] 2009 NRCT 荷兰 24 波生坦 38.0(NA) ASD(58.3%)
VSD(29.2%)
Com(12.5%)是(100%) 3 NA 6MWD Gatzoulis等[17] 2019 RCT 英国 114 马西替坦 33.0(12.8) NA 是(17.5%) 4 2.4(0.5) 6MWD Zuckerman等[18] 2011 NRCT 美国 17 安立生坦 32.2(11.9) ASD(52.9%)
VSD(41.2%)
Com(5.9%)是(17.6%) 30 NA 6MWD PDE5i Zhang等[19] 2011 NRCT 中国 84 西地那非 28.0(9.0) ASD(29.8%)
VSD(40.5%)
PDA(27.4%)
Com(2.4%)否 12 2.6(0.7) 6MWD Chua等[20] 2007 NRCT 中国 7 西地那非 37.0(11.0) ASD(71.4%)
VSD(28.6%)否 6 3.3(0.7) 6MWD Bharani等[21] 2007 RCT 印度 8 他达那非 28.0(9.4) NA 否 1 NA 6MWD Mukhopadhyay等[22] 2011 RCT 印度 28 他达那非 29.3(11.7) ASD(50.0%)
VSD(46.4%)
Com(3.6%)否 1.5 2.2(0.4) 6MWD Chon等[23] 2017 NRCT 韩国 11 伊洛前列素 44.2(12.2) ASD(9.1%)
VSD(54.5%)
PDA(27.3%)
Com(9.1%)否 12 3.4(0.5) 6MWD Cha等[24] 2013 NRCT 韩国 13 伊洛前列素 45.0(11.0) ASD(30.8%)
VSD(61.5%)
PDA(23.1%)
Com(15.4%)否 6 3.3(0.5) 6MWD Fernandes等[25] 2003 NRCT 美国 8 依前列醇 36.3(14.9) ASD(37.5%)
VSD(25.0%)
PDA(12.5%)
Com(12.5%)否 3 3.8(0.4) 6MWD 表 3 纳入随机对照试验文献质量评价*
Table 3. Quality assessment of randomized controlled trials
纳入研究 随机分配方案产生 分配方案隐藏 盲法 数据结果完整 未选择性报告结果 无明显其他偏倚 文献质量 Gali等[9] A A A A A A H Gatzoulis等[17] A B A A A A M Bharani等[21] B B A A A B M Mukhopadhyay等[22] B B A A A B M *质量评价采用Cochrane ROB评价工具评估,各评价条目分为A(是)、B(不清楚)、C(否)3级,表示为低风险、中等风险及高风险。若所有评价条目均为低风险,则文献质量评价为高质量;若有一个条目或多个条目为中等风险,则文献质量评价为中等质量;若其中一个条目或多个条目为高风险,则文献质量评价为低质量。 表 4 纳入队列研究文献质量评价*
Table 4. Evaluation of literature quality of included cohort studies
纳入研究 (队列)选择 可比性§ 结局 评分 暴露队列的代表性 非暴露队列的选择 暴露资料的来源 证实研究初始无观察结果 结局评价 随访时限是否足够 队列随访适当# Crepaz等[10] 1 - 1 1 1 1 1 1 7 Serino等[11] 1 - 1 1 1 1 1 - 6 张宏伟等[12] 1 - 1 1 2 1 - - 6 D’alto等[13] 1 - 1 1 2 1 - 1 7 Kermeen等[14] 1 - 1 1 1 1 1 1 7 Kaya等[15] 1 - 1 1 2 1 1 1 8 Duffels等[16] 1 - 1 1 1 1 - 1 6 Zuckerman等[18] 1 - 1 1 2 1 1 1 8 Zhang等[19] 1 - 1 1 1 1 - 1 6 Chau等[20] 1 1 1 1 1 1 - 1 7 Chon等[23] 1 - 1 1 1 1 - 1 6 Cha等[24] 1 - 1 1 1 1 - 1 6 Fernandes等[25] 1 - 1 1 1 1 - 1 6 *非随机对照试验质量评价采用Newcastle-Ottawa(NOS)队列研究质量评价清单,总分定义为9分,≥7分提示低风险;§可比性:1)研究控制单一靶向药物治疗PAH;2)研究控制了任何其他混杂因素(控制纳入对象未合并其他遗传代谢性疾病,如DS)。#失访率<5%; -
[1] Wood P. The Eisenmenger syndrome or pulmonary hypertension with reversed central shunt[J]. Br Med J, 1958, 2(5098): 701-709. doi: 10.1136/bmj.2.5098.701
[2] Gupta R, Shore S, Rodriguez MM, et al. Eisenmenger syndrome[J]. Principles of Pulmonary Protection in Heart Surgery, 2010, 9(5): 171-177.
[3] Galie N, Manes A, Palazzini M, et al. Management of pulmonary arterial hypertension associated with congenital systemic-to-pulmonary shunts and Eisenmenger's syndrome[J]. Drugs, 2008, 68(8): 1049-1066. doi: 10.2165/00003495-200868080-00004
[4] Elshafay A, Truong DH, Aboelnas MM, et al. The effect of endothelin receptor antagonists in patients with Eisenmenger syndrome: A systematic review[J]. Am J of Cardiovasc Drugs Devices, 2017, 18(21): 1-10.
[5] Opitz C, Rosenkranz S, Ghofrani H A, et al. ESC guidelines 2015 pulmonary hypertension: diagnosis and treatment[J]. Deut Med Wochens, 2016, 141(24): 1764-1770. doi: 10.1055/s-0042-117784
[6] 王利婷, 荆志成. 肺动脉高压治疗药物研究进展[J]. 国际药学研究杂志, 2017, 44(2): 127-134. https://www.cnki.com.cn/Article/CJFDTOTAL-GWYZ201702013.htm
[7] Li Q, Kuang HY, Lu TW, et al. What is the position of PAH-specific drug therapy inpatients with Eisenmenger syndrome: A systematic review and meta-analysis[J]. Medicine, 2019, 98(20): e15632. doi: 10.1097/MD.0000000000015632
[8] Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomized trials[J]. BMJ, 2011, 343: d5928. doi: 10.1136/bmj.d5928
[9] Galiã N, Beghetti M, Gatzoulis MA, et al. Bosentan therapy in patients with Eisenmenger syndrome: a multicenter, double-blind, randomized, placebo-controlled study[J]. Circulation, 2006, 114(1): 48-54. doi: 10.1161/CIRCULATIONAHA.106.630715
[10] Crepaz R, Romeo C, Montanaro D, et al. Long-term results of treatment with bosentan in adult Eisenmenger's syndrome patients with Down's syndrome related to congenital heart disease[J]. BMC Cardiovasc Disor, 2013, 13(74): 1-7.
[11] Serino G, Guazzi M, Micheletti A, et al. Effect of bosentan on exercise capacity and clinical worsening in patients with dual Down and Eisenmenger syndrome[J]. Clin Med Insights Cardiol, 2013, 7(7): 29-34.
[12] 张宏伟, 顾虹. 波生坦治疗儿童艾森曼格综合征的临床观察[J]. 中国医药, 2013, 8(4): 459-460. doi: 10.3760/cma.j.issn.1673-4777.2013.04.010
[13] D'Alto M, Vizza CD, Romeo E, et al. Long-term effects of bosentan treatment in adult patients with pulmonary arterial hypertension related to congenital heart disease(Eisenmenger physiology): safety, tolerability, clinical, and hemodynamic effect[J]. Heart, 2007, 93(5): 621-625. doi: 10.1136/hrt.2006.097360
[14] Kermeen FD, Franks C, O'Brien K, et al. Endothelin receptor antagonists are an effective long-term treatment option in pulmonary arterial hypertension associated with congenital heart disease with or without trisomy-21[J]. Heart Lung Circ, 2010, 19(10): 595-600. doi: 10.1016/j.hlc.2010.07.005
[15] Kaya MG, Lam YY, Erer B, et al. Long-term effect of bosentan therapy on cardiac function and symptomatic benefits in adult patients with Eisenmenger syndrome[J]. J Card Fail, 2012, 18(5): 379-382. doi: 10.1016/j.cardfail.2012.02.004
[16] Duffels MG, Vis JC, van Loon RL, et al. Down patients with Eisenmenger syndrome: is bosentan treatment an option?[J]. Int J Cardiol, 2009, 134(3): 378-383. doi: 10.1016/j.ijcard.2008.02.025
[17] Gatzoulis MA, Landzberg M, Beghetti M, et al. Evaluation of macitentan in patients with Eisenmenger syndrome: results from the randomized, controlled MAESTRO study[J]. Circulation, 2019, 139(1): 51-63. doi: 10.1161/CIRCULATIONAHA.118.033575
[18] Zuckerman WA, Leaderer D, Rowan CA, et al. Ambrisentan for pulmonary arterial hypertension due to congenital heart disease[J]. Am J Cardiol, 2011, 107(9): 1381-1385. doi: 10.1016/j.amjcard.2010.12.051
[19] Zhang ZN, Jiang X, Zhang R, et al. Oral sildenafil treatment for Eisenmenger syndrome: a prospective, open-label, multicentre study[J]. Heart, 2011, 97(22): 1876-1881. doi: 10.1136/heartjnl-2011-300344
[20] Chua EM, Fan KY, Chow WH. Effects of chronic sildenafil in patients with Eisenmenger syndrome versus idiopathic pulmonary arterial hypertension[J]. Int J Cardiol, 2007, 120(3): 301-305. doi: 10.1016/j.ijcard.2006.10.018
[21] Bharani A, Patel A, Saraf J, et al. Efficacy and safety of PDE-5 inhibitor tadalafil in pulmonary arterial hypertension[J]. Indian Heart J, 2007, 59(4): 323-328.
[22] Mukhopadhyay S, Nathani S, Yusuf J, et al. Clinical efficacy of phosphodiesterase-5 inhibitor tadalafil in Eisenmenger syndrome-randomized, placebo-controlled, double[J]. Congenit Heart Dis, 2011, 6(5): 424-431. doi: 10.1111/j.1747-0803.2011.00561.x
[23] Chon MK, Cho KI, Cha KS, et al. Effects of long-term iloprost treatment on right ventricular function in patients with Eisenmenger syndrome[J]. J Cardiol, 2017, 69(5): 741-746. doi: 10.1016/j.jjcc.2016.07.002
[24] Cha KS, Cho KI, Seo JS, et al. Effects of inhaled iloprost on exercise capacity, quality of life, and cardiac function in patients with pulmonary arterial hypertension secondary to congenital heart disease(the Eisenmenger syndrome)(from the EIGER Study)[J]. Am J Cardiol, 2013, 112(11): 1834-1839. doi: 10.1016/j.amjcard.2013.08.003
[25] Fernandes SM, Newburger JW, Lang P, et al. Usefulness of epoprostenol therapy in the severely ill adolescent/adult with Eisenmenger physiology[J]. Am J Cardiol, 2003, 91(5): 632-635. doi: 10.1016/S0002-9149(02)03328-3
[26] Simonneau G, Gatzoulis M, Adatia I, et al. Updated clinical classification of pulmonary hypertension[J]. J Am Coll Cardiol. 2013;62(25): 34-41. doi: 10.1016/j.jacc.2013.10.029
[27] Diller GP, Körten MA, Bauer UM, et al. Current therapy and outcome of Eisenmenger syndrome: data of the German National Register for congenital heart defects[J]. Eur Heart J, 2016, 37(18): 1449-1455. doi: 10.1093/eurheartj/ehv743
[28] Vongpatanasin W, Brickner ME, Hillis LD, et al. The Eisenmenger syndrome in adults[J]. Ann Intern Med, 1998, 128(9): 745-755. doi: 10.7326/0003-4819-128-9-199805010-00008
[29] Duo-Ji MM, Long ZW. Comparative efficacy and acceptability of endothelin receptor antagonists for pulmonary arterial hypertension: A network meta-analysis[J]. Int J Cardiol, 2017, 234: 90-98. doi: 10.1016/j.ijcard.2016.12.092
[30] Raja SG, Danton MD, MacArthur KJ, et al. Treatment of pulmonary arterial hypertension with sildenafil: from pathophysiology to clinical evidence[J]. J Cardiothorac Vasc Anesth, 2006, 20(5): 722-735. doi: 10.1053/j.jvca.2005.12.011
[31] Opitz CF, Wensel R, Winkler J, et al. Clinical efficacy and survival with first-line inhaled iloprost therapy in patients with idiopathic pulmonary arterial hypertension[J]. Eur Heart J, 2005, 26(18): 1895-1902. doi: 10.1093/eurheartj/ehi283
[32] Humbert M, Segal ES, Kiely DG, et al. Results of European post-marketing surveillance of bosentan in pulmonary hypertension[J]. Eur Respir J, 2007, 30(2): 338-344. doi: 10.1183/09031936.00138706
[33] Hascoët S, Baruteau AE, Humbert M, et al. Long-term outcomes of pulmonary arterial hypertension under specific drug therapy in Eisenmenger syndrome[J]. J Heart Lung Transplant, 2017, 36(4): 386-398. doi: 10.1016/j.healun.2016.10.006
[34] 梁富翔, 宋兵, 祁泉, 等. 内皮素受体拮抗剂治疗肺动脉高压疗效和耐受性的网状Meta分析[J]. 中国循证心血管医学杂志, 2014, 10(6): 663-669. https://www.cnki.com.cn/Article/CJFDTOTAL-PZXX201406004.htm
[35] 牛力, 潘家义, 谌晶晶, 等. 安立生坦与波生坦联合西地那非治疗先天性心脏病合并中重度肺动脉高压疗效的对比研究[J]. 临床心血管病杂志, 2021, 37(4): 346-350. http://lcxb.cbpt.cnki.net/WKC/WebPublication/paperDigest.aspx?paperID=b757adb7-e912-46aa-a611-422036437c62
-




 下载:
下载: