Meta-analysis and dose-response analysis of the relationship between plasma trimethylamine N-oxide level and atrial fibrillation
-
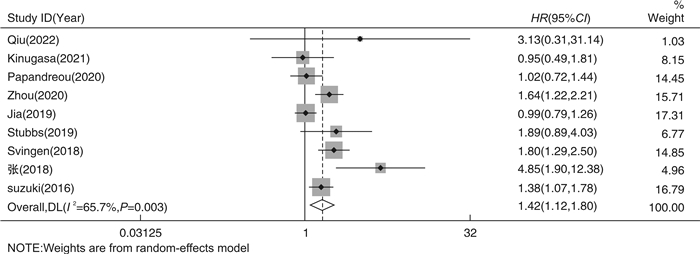
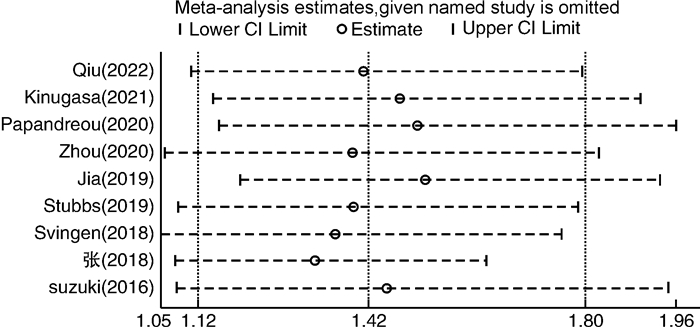
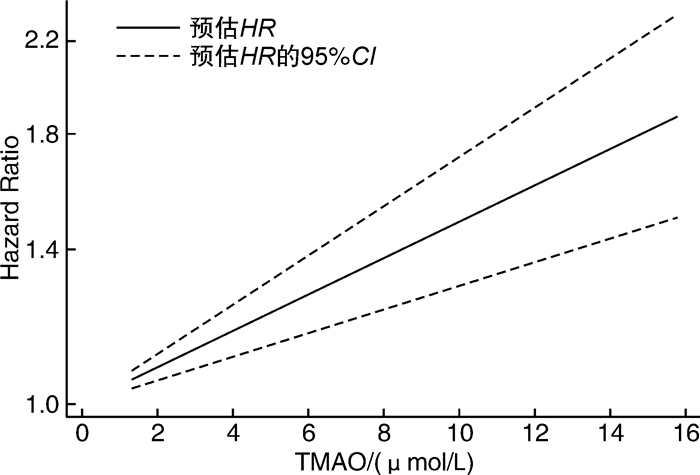
摘要: 目的 采用meta分析和剂量-反应关系评价血浆氧化三甲胺水平(TMAO)与心房颤动(AF)发病风险之间的关系。方法 通过计算机搜索中国知网、万方数据库、Pubmed、Embase、Cochrane Library、Web of Science等公开发表的TMAO水平与AF发生的前瞻性队列研究。检索时限从建库至2023年3月,通过纽卡斯尔-渥太华量表(NOS)进行质量评价后提取纳入文献的相关数据,采用Stata 17.0软件进行meta分析及剂量-反应关系分析。结果 共纳入9篇文献,总计531 970例研究对象。Meta分析结果显示,TMAO水平与AF发生的风险显著相关(HR=1.42,95%CI 1.12~1.80)。剂量-反应分析显示,血浆TMAO水平与AF发生的风险呈线性剂量-反应关系,血浆TMAO水平每升高1 μmol/L,AF发生的风险增加4.06%。结论 血浆TMAO水平与AF发生的风险显著相关。血浆TMAO水平每升高1 μmol/L,AF发生的风险增加4.06%。Abstract: Objective Meta analysis and a dose-response relationship system were used to assess the relationship between plasma trimethylamine N-oxide(TMAO) level and atrial fibrillation.Methods Databases including CNKI, WanFang Data, PubMed, Embase, Cochrane Library, Web of Science were searched for prospective cohort studies on the relationship between plasma TMAO level and the risk of atrial fibrillation from the establishment of the database to March 2023. The data of the included literatures were extracted, the Newcastle-Ottawa Scale(NOS) was used to evaluate the quality of the included literatures, and Stata 17.0 was used for meta-analysis and dose-response relationship analysis.Results This study included a total of 9 articles with a total of 531 970 subjects. The findings of the meta-analysis revealed that the level of TMAO was significantly related to the risk of AF(HR=1.42, 95%CI 1.12-1.80). Dose-response analysis showed that there was a linear dose-response relationship between plasma TMAO level and the risk of AF. The risk of AF increased by 4.06% for every 1 μmol/L increase in plasma TMAO level.Conclusion There was a significant correlation between plasma TMAO level and the risk of AF. The risk of AF increased by 4.06% for every 1 μmol/L increase in plasma TMAO level.
-
Key words:
- trimethylamine-N-oxide /
- atrial fibrillation /
- meta-analysis /
- dose-response analysis
-

-
表 1 纳入文献的基本特征和NOS评分
Table 1. Basic characteristics and NOS scores of the included literatures
X±S, M(P25, P75) 第1作者 发表时间/年 国家 研究对象 样本量/例 平均年龄/岁 男性/% TMAO/(μmol/L) AF/% 糖尿病/% 高血压/% NOS评分 Qiu[13] 2022 中国 缺血性心力衰竭 189 64±10.5 83.1 4.92(2.55,6.84) 4.8 33.3 52.4 9 Kinugasa[14] 2021 日本 急性心力衰竭 146 80(73,85) 46.4 20.37(10.45,38.31) 50 40.4 81.8 9 Papandreou[15] 2020 西班牙 AF 1 127 68.4 50.6 — 45.2 47.9 88.4 9 Zhou[16] 2020 中国 心肌梗死后慢性心力衰竭 1 208 73(64,80) 68.5 4.5 18.6 28.6 44.7 8 Jia[17] 2019 AFGen(多国) AF 522 744 — — — 12.5 — — 7 Stubbs[18] 2019 多国 ESKD 1 243 54±14 60 2.5~1103.1 6 32 92 9 Svingen[19] 2018 挪威 疑似稳定型心绞痛 4 141 62(51,73) 71.9 7.57(2.2~23.5) 8.3 11.8 46.7 8 张[20] 2018 中国 冠心病合并房颤与单纯冠心病 200 62.7 54.5 5.02 70 14 34.5 8 Suzuki[21] 2016 英国 急性心力衰竭 972 78(69,84) 61 5.6(3.4~10.5) 45.4 33.8 58.3 8 注:ESKD:终末期肾病。 表 2 TMAO与AF发生的亚组分析
Table 2. Subgroup analysis of TMAO and AF occurrence
亚组 HR(95%CI) 占比/% I2/% P 发表年份 2020年及以后 1.25(0.89~1.78) 39.33 47.1 0.129 2020年以前 1.59(1.11~2.28) 60.67 77.3 0.001 国家 中国 2.55(1.10~5.91) 21.7 59.1 0.087 非中国 1.26(1.00~1.58) 78.3 58.9 0.033 研究对象 心力衰竭 1.44(1.20~1.73) 41.67 0 0.418 非心力衰竭 1.52(1.01~2.28) 58.33 79 0.001 样本量 ≤1 000例 1.72(0.92~3.22) 30.92 64.6 0.036 >1 000例 1.35(1.01~1.81) 69.08 71.6 0.007 研究对象平均年龄 ≤65岁 2.18(1.43~3.32) 27.6 24.8 0.263 >65岁 1.30(1.03~1.63) 55.09 43 0.154 N/A 0.99(0.78~1.25) 17.31 — — TMAO水平 ≤5.5 μmol/L 2.55(1.10~5.91) 21.7 59.1 0.087 >5.5 μmol/L 1.49(1.18~1.87) 46.55 21.2 0.283 N/A 1.00(0.82~1.21) 31.75 0 0.889 男性占比 ≤60% 1.57(0.85~2.89) 34.33 73.2 0.011 >60% 1.56(1.32~1.85) 48.37 0 0.558 N/A 0.99(0.78~1.25) 17.31 — — 糖尿病例数占比 ≤33% 1.90(1.42~2.54) 42.28 36 0.196 >33% 1.21(0.99~1.49) 40.41 4.2 0.372 N/A 0.99(0.78~1.25) 17.31 — — 高血压例数占比 ≤80% 1.72(1.32~2.24) 53.33 46.6 0.112 >80% 1.21(0.80~1.55) 29.36 14.9 0.309 N/A 0.99(0.78~1.25) 17.31 — — -
[1] Sagris M, Vardas EP, Theofilis P, et al. Atrial fibrillation: pathogenesis, predisposing factors, and genetics[J]. Int J Mol Sci, 2021, 23(1): 6. doi: 10.3390/ijms23010006
[2] Wei Y, Zhou G, Wu X, et al. Latest incidence and electrocardiographic predictors of atrial fibrillation: a prospective study from China[J]. Chin Med J(Engl), 2023, 136(3): 313-321.
[3] Zhang L, Liu Y, Wang X, et al. Physical exercise and diet: regulation of gut microbiota to prevent and treat metabolic disorders to maintain health[J]. Nutrients, 2023, 15(6): 1539. doi: 10.3390/nu15061539
[4] Zhang B, Wang X, Xia R, et al. Gut microbiota in coronary artery disease: a friend or foe?[J]. Biosci Rep, 2020, 40(5): BSR20200454. doi: 10.1042/BSR20200454
[5] Avery EG, Bartolomaeus H, Maifeld A, et al. The gut microbiome in hypertension: recent advances and future perspectives[J]. Circ Res, 2021, 128(7): 934-950. doi: 10.1161/CIRCRESAHA.121.318065
[6] Iatcu CO, Steen A, Covasa M. Gut microbiota and complications of type-2 diabetes[J]. Nutrients, 2021, 14(1): 166. doi: 10.3390/nu14010166
[7] Rebersek M. Gut microbiome and its role in colorectal cancer[J]. BMC Cancer, 2021, 21(1): 1325. doi: 10.1186/s12885-021-09054-2
[8] Rashid S, Noor TA, Saeed H, et al. Association of gut microbiome dysbiosis with the progression of atrial fibrillation: A systematic review[J]. Ann Noninvasive Electrocardiol, 2023: e13059.
[9] Tabata T, Yamashita T, Hosomi K, et al. Gut microbial composition in patients with atrial fibrillation: effects of diet and drugs[J]. Heart Vessels, 2021, 36(1): 105-114. doi: 10.1007/s00380-020-01669-y
[10] Huang R, Yan L, Lei Y. The gut microbial-derived metabolite trimethylamine N-oxide and atrial fibrillation: relationships, mechanisms, and therapeutic strategies[J]. Clin Interv Aging, 2021, 16: 1975-1986. doi: 10.2147/CIA.S339590
[11] Melnychuk I, Lizogub VG. Gut microbiota composition and its metabolites changes in patients with atherosclerosis and atrial fibrillation[J]. Wiad Lek, 2022, 75(12): 2994-2999. doi: 10.36740/WLek202212117
[12] Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses[J]. Eur J Epidemiol, 2010, 25(9): 603-605. doi: 10.1007/s10654-010-9491-z
[13] Qiu WD, Xiao XJ, Xia S, et al. Predictive value of plasma TMAO combined with NT-proBNP on the prognosis and length of hospitalization of patients with ischemic heart failure[J]. Zhonghua Xin Xue Guan Bing Za Zhi, 2022, 50(7): 684-689.
[14] Kinugasa Y, Nakamura K, Kamitani H, et al. Trimethylamine N-oxide and outcomes in patients hospitalized with acute heart failure and preserved ejection fraction[J]. ESC Heart Fail, 2021, 8(3): 2103-2110. doi: 10.1002/ehf2.13290
[15] Papandreou C, Bulló M, Hernández-Alonso P, et al. Choline metabolism and risk of atrial fibrillation and heart failure in the PREDIMED Study[J]. Clin Chem, 2021, 67(1): 288-297. doi: 10.1093/clinchem/hvaa224
[16] Zhou X, Jin M, Liu L, et al. Trimethylamine N-oxide and cardiovascular outcomes in patients with chronic heart failure after myocardial infarction[J]. ESC Heart Fail, 2020, 7(1): 188-193.
[17] Jia J, Dou P, Gao M, et al. Assessment of causal direction between gut microbiota-dependent metabolites and cardiometabolic health: a bidirectional mendelian randomization analysis[J]. Diabetes, 2019, 68(9): 1747-1755. doi: 10.2337/db19-0153
[18] Stubbs JR, Stedman MR, Liu S, et al. Trimethylamine N-Oxide and cardiovascular outcomes in patients with eskd receiving maintenance hemodialysis[J]. Clin J Am Soc Nephrol, 2019, 14(2): 261-267. doi: 10.2215/CJN.06190518
[19] Svingen G, Zuo H, Ueland PM, et al. Increased plasma trimethylamine-N-oxide is associated with incident atrial fibrillation[J]. Int J Cardiol, 2018, 267: 100-106. doi: 10.1016/j.ijcard.2018.04.128
[20] 张兰玉, 张佩生. 肠道菌群代谢产物氧化三甲胺与房颤发生的关系[J]. 世界复合医学, 2018, 4(5): 30-33. https://www.cnki.com.cn/Article/CJFDTOTAL-SJFH201805012.htm
[21] Suzuki T, Heaney LM, Bhandari SS, et al. Trimethylamine N-oxide and prognosis in acute heart failure[J]. Heart, 2016, 102(11): 841-848. doi: 10.1136/heartjnl-2015-308826
[22] Thomas MS, Fernandez ML. Trimethylamine N-Oxide(TMAO), diet and cardiovascular disease[J]. Curr Atheroscler Rep, 2021, 23(4): 1212.
[23] Gatarek P, Kaluzna-Czaplinska J. Trimethylamine N-oxide(TMAO)in human health[J]. Excli J, 2021, 20: 301-319.
[24] 杨敏, 肖模超. 心房颤动患者左心房形态结构及功能与血栓形成关系的研究进展[J]. 临床心血管病杂志, 2023, 39(2): 103-107. doi: 10.13201/j.issn.1001-1439.2023.02.006 https://lcxxg.whuhzzs.com/article/doi/10.13201/j.issn.1001-1439.2023.02.006
[25] Li Z, Wu Z, Yan J, et al. Gut microbe-derived metabolite trimethylamine N-oxide induces cardiac hypertrophy and fibrosis[J]. Lab Invest, 2019, 99(3): 346-357. doi: 10.1038/s41374-018-0091-y
[26] Yang W, Zhao Q, Yao M, et al. The transformation of atrial fibroblasts into myofibroblasts is promoted by trimethylamine N-oxide via the Wnt3a/β-catenin signaling pathway[J]. J Thorac Dis, 2022, 14(5): 1526-1536. doi: 10.21037/jtd-22-475
[27] 靳步, 纪方方, 左安俊, 等. 氧化三甲胺通过促进成年小鼠心肌细胞T小管重构加重心力衰竭[J]. 中国病理生理杂志, 2020, 36(6): 1034-1041. https://www.cnki.com.cn/Article/CJFDTOTAL-ZBLS202006012.htm
[28] Jiang WY, Huo JY, Wang SC, et al. Trimethylamine N-oxide facilitates the progression of atrial fibrillation in rats with type 2 diabetes by aggravating cardiac inflammation and connexin remodeling[J]. J Physiol Biochem, 2022, 78(4): 855-867. doi: 10.1007/s13105-022-00908-2
[29] Konieczny R, urawska-Płaksej E, Kaaz K, et al. All-cause mortality and trimethylamine N-Oxide levels in patients with cardiovascular disease[J]. Cardiology, 2022, 147(4): 443-452. doi: 10.1159/000525972
[30] Sanchez-Gimenez R, Ahmed-Khodja W, Molina Y, et al. Gut microbiota-derived metabolites and cardiovascular disease risk: a systematic review of prospective cohort studies[J]. Nutrients, 2022, 14(13): 2654. doi: 10.3390/nu14132654
[31] Luciani M, Müller D, Vanetta C, et al. Trimethylamine-N-oxide is associated with cardiovascular mortality and vascular brain lesions in patients with atrial fibrillation[J]. Heart, 2023, 109(5): 396-404.
[32] Gong D, Zhang L, Zhang Y, et al. Gut microbial metabolite trimethylamine N-Oxide is related to thrombus formation in atrial fibrillation patients[J]. Am J Med Sci, 2019, 358(6): 422-428. doi: 10.1016/j.amjms.2019.09.002
-




 下载:
下载: