Association of residual cholesterol with early-onset myocardial infarction and its clinical outcome
-
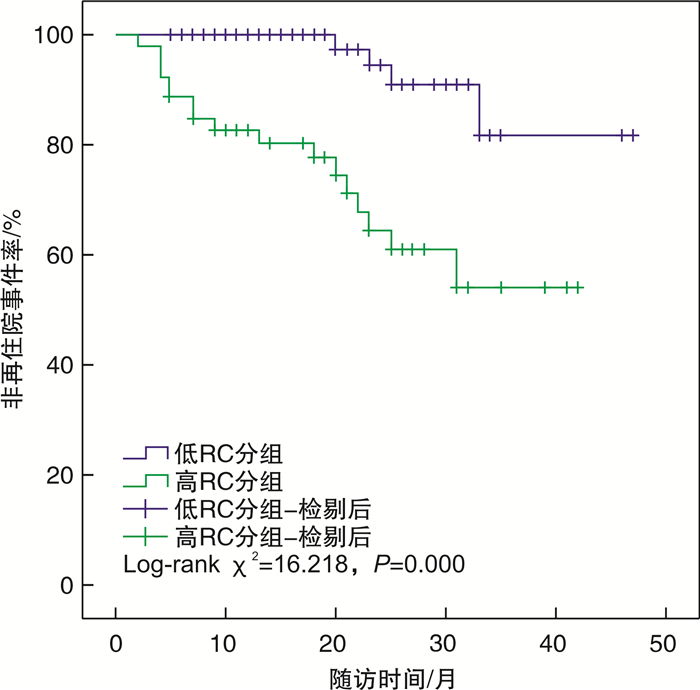
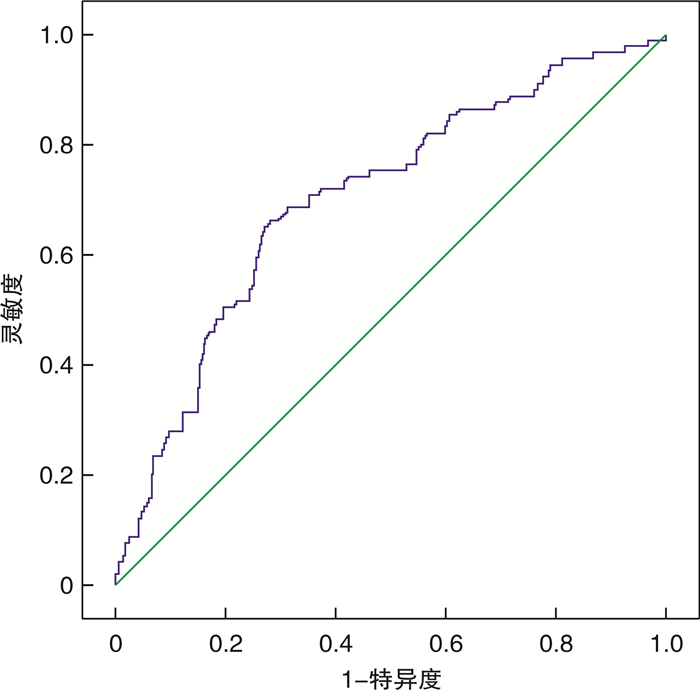
摘要: 目的 观察残余胆固醇(RC)与早发心肌梗死(MI)及其临床结局的相关性。方法 连续纳入2019年1月—2022年8月于沧州市人民医院住院首次诊断为MI的患者637例,并依据发病年龄(男性≤50岁,女性≤60岁)分为早发MI组(129例)和非早发MI组(508例),所有患者均接受经皮冠状动脉介入治疗和冠心病规律药物治疗,并将早发MI患者依据随访期间是否再住院分为再住院组(21例)和非再住院组(108例)。收集患者一般临床资料和总胆固醇(TC)、甘油三酯(TG)、高密度脂蛋白胆固醇(HDL-C)及低密度脂蛋白胆固醇(LDL-C)等指标,并根据公式计算出RC。比较组间患者的临床特征,采用多因素logistic回归分析早发MI的影响因素。绘制受试者工作特征曲线(ROC),评估RC对早发MI患者再住院的预测价值。Kaplan-Meier生存曲线比较组间再住院事件率的差异。多因素Cox比例风险回归分析早发MI患者再住院的影响因素,确定RC与再住院的关系。结果 早发MI组TC、LDL-C、RC、尿酸水平均高于非早发MI组,均差异有统计学意义(P < 0.05)。再住院组TC、TG、RC水平均高于非再住院组,均差异有统计学意义(P < 0.05)。多因素logistic回归分析结果显示,尿酸(OR=1.002,95%CI 1.000~1.004,P=0.026)、LDL-C(OR=3.031,95%CI 1.253~7.333,P=0.014)和RC(OR=2.856,95%CI 1.253~6.507,P=0.013)均为早发MI的独立危险因素。RC预测早发MI患者再住院的ROC曲线下面积(AUC)为0.703(95%CI 0.644~0.762,P=0.000)。高RC组和低RC组再住院率比较,差异有统计学意义(Log-rank χ2=16.218,P=0.000)。多因素Cox回归分析结果显示,LDL-C(HR=23.905,95%CI 1.546~369.646,P=0.023)、RC(HR=29.837,95%CI 1.976~450.493,P=0.014)和TG(HR=2.045,95%CI 1.458~2.869,P=0.000)均为早发MI患者再住院的独立危险因素。结论 高RC是早发MI和预后不良的独立危险因素。Abstract: Objective To observe the association of residual cholesterol(RC) with early-onset myocardial infarction(MI) and its clinical outcomes.Methods A total of 637 patients who were hospitalized in Cangzhou People's Hospital for the first time from January 2019 to August 2022 were continuously included. According to the age of onset(male≤50 years old, female≤60 years old), it was divided into early-onset MI group(129 cases) and non-early-onset MI group(508 cases), and all patients received percutaneous coronary intervention and regular drug treatment for coronary heart disease, and the early-onset MI were divided into rehospitalized group(21 cases) and non-rehospitalized group(108 cases) according to whether they were rehospitalized during the follow-up period. The clinical characteristics of patients were compared between groups, and the influencing factors of early-onset MI were analyzed by multivariate logistic regression. Receiver operating characteristic curve(ROC) was plotted to evaluate the predictive value of RC for readmission in patients with early-onset MI. Kaplan-Meier survival curve was used to compare the rate of readmission events between the groups. Multivariate Cox proportional risk regression analysis was conducted to determine the relationship between RC and readmission in patients with early-onset MI.Results The levels of TC, LDL-C, RC and uric acid in the early-onset MI group were higher than those in the non-early-onset MI group, and the differences were statistically significant(P < 0.05). The levels of TC, TG and RC in the rehospitalization group were higher than those in the nonrehospitalization group(P < 0.05). The results of multivariate logistic regression analysis showed that uric acid(OR=1.002, 95%CI1.000-1.004, P=0.026), LDL-C(OR=3.031, 95%CI1.253-7.333, P=0.014) and RC(OR=2.856, 95%CI1.253-6.507, P=0.013) were independent risk factors for early-onset MI. AUC for RC predicting rehospitalization in patients with early-onset MI was 0.703(95%CI0.644-0.762, P=0.000). There were significant differences in the rehospitalization rates between the high RC group and the low RC group(Log-rank χ2=16.218, P=0.000). The results of multivariate Cox regression analysis showed that LDL-C(HR=23.905, 95%CI1.546-369.646, P=0.023), RC(HR=29.837, 95%CI1.976-450.493, P=0.014) and TG(HR=2.045, 95%CI1.458-2.869, P=0.000) were independent risk factors for rehospitalization of patients with early-onset MI.Conclusion High RC is an independent risk factor for early-onset MI risk and poor prognosis.
-

-
表 1 早发MI组和非早发MI组临床资料
Table 1. Clinical data of early-onset MI group and non-early-onset MI group
例(%), X±S, M(P25, P75) 指标 早发MI组(129例) 非早发MI组(508例) χ2/t/Z P值 男性 90(69.77) 347(68.31) 0.102 0.750 吸烟史 34(26.36) 132(25.98) 0.007 0.931 饮酒史 30(23.26) 106(20.87) 0.350 0.554 高血压史 69(53.49) 308(60.63) 2.172 0.141 糖尿病史 26(20.16) 130(25.59) 1.644 0.200 白蛋白/(g/L) 42.01±4.01 42.04±3.96 0.455 0.932 肌酐/(μmol/L) 61.75±10.51 61.97±11.40 1.901 0.842 尿酸/(μmol/L) 307.42±103.80 287.19±101.03 0.697 0.044 谷丙转氨酶/(U/L) 21.67±13.42 20.25±12.26 2.884 0.247 TC/(mmol/L) 4.98±1.24 4.54±0.98 3.424 0.000 TG/(mmol/L) 1.96±1.22 1.80±1.34 0.006 0.216 HDL-C/(mmol/L) 1.09±0.27 1.12±0.29 1.542 0.454 LDL-C/(mmol/L) 2.98(2.43,3.86) 2.79(2.22,3.34) 5.432 0.001 RC/(mmol/L) 0.72±0.73 0.61±0.43 2.798 0.025 表 2 早发MI的多因素logisitic回归分析
Table 2. Multivariate logisitic regression analysis of early onset MI
变量 B SE Wald OR 95%CI P 尿酸 0.002 0.001 4.982 1.002 1.000~1.004 0.026 LDL-C 1.109 0.451 6.055 3.031 1.253~7.333 0.014 RC 1.049 0.420 6.235 2.856 1.253~6.507 0.013 表 3 再住院组和非再住院组临床资料
Table 3. Clinical data of readmission group and non-readmission group
例(%), X±S, M(P25, P75) 指标 再住院组(21例) 非再住院组(108例) χ2/t/Z P值 年龄/岁 43.76±8.34 46.44±7.15 1.409 0.129 男性 14(66.67) 76(70.37) 0.114 0.735 吸烟史 5(23.81) 29(26.85) 0.084 0.772 饮酒史 5(23.81) 25(23.15) 0.004 0.948 高血压史 10(47.62) 59(54.63) 0.347 0.556 糖尿病史 5(23.81) 21(19.44) 0.208 0.648 白蛋白/(g/L) 41.95±3.56 42.02±4.09 0.682 0.946 肌酐/(μmol/L) 62.71±10.00 61.58±10.63 0.319 0.669 尿酸/(μmol/L) 321.84±114.09 304.93±02.27 0.676 0.514 谷丙转氨酶/(U/L) 22.95±15.51 21.46±13.09 0.941 0.656 TC/(mmol/L) 5.95(4.70,6.38) 4.63(4.16,5.37) 6.356 0.007 TG/(mmol/L) 2.73(1.77,3.65) 1.51(1.11,2.03) 10.385 0.004 HDL-C/(mmol/L) 1.07±0.34 1.10±0.26 1.795 0.632 LDL-C/(mmol/L) 3.39(2.53,4.48) 2.93(2.42,3.74) 10.161 0.185 RC/(mmol/L) 1.10(0.68,1.42) 0.55(0.38,0.74) 30.343 0.017 表 4 早发MI患者再住院的多因素Cox回归分析
Table 4. Multivariate Cox regression analysis of re-hospitalization in early MI
变量 B SE Wald HR 95%CI P LDL-C 3.174 1.397 5.161 23.905 1.546~369.646 0.023 RC 3.396 1.385 6.011 29.837 1.976~450.493 0.014 TG 0.716 0.173 17.172 2.045 1.458~2.869 0.000 -
[1] Emerging Risk Factors Collaboration, Di Angelantonio E, Sarwar N, et al. Major lipids, apolipoproteins, and risk of vascular disease[J]. JAMA, 2009, 302(18): 1993-2000. doi: 10.1001/jama.2009.1619
[2] Gordon DJ, Probstfield JL, Garrison RJ, et al. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies[J]. Circulation, 1989, 79(1): 8-15. doi: 10.1161/01.CIR.79.1.8
[3] Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update From the GBD 2019 Study[J]. J Am Coll Cardiol, 2020, 76(25): 2982-3021. doi: 10.1016/j.jacc.2020.11.010
[4] Dhindsa DS, Sandesara PB, Shapiro MD, et al. The evolving understanding and approach to residual cardiovascular risk management[J]. Front Cardiovasc Med, 2020, 7: 88. doi: 10.3389/fcvm.2020.00088
[5] Langsted A, Nordestgaard BG. Nonfasting versus fasting lipid profile for cardiovascular risk prediction[J]. Pathology, 2019, 51(2): 131-141. doi: 10.1016/j.pathol.2018.09.062
[6] Langsted A, Madsen CM, Nordestgaard BG. Contribution of remnant cholesterol to cardiovascular risk[J]. J Intern Med, 2020, 288(1): 116-127. doi: 10.1111/joim.13059
[7] 邓毅凡, 朱米雪, 刘娟, 等. 残粒脂蛋白-胆固醇与早发冠心病及冠状动脉病变程度的相关性[J]. 临床心血管病杂志, 2022, 38(7): 536-540. https://lcxxg.whuhzzs.com/article/doi/10.13201/j.issn.1001-1439.2022.07.005
[8] Nazli SA, Chua YA, Mohd Kasim NA, et al. Familial hypercholesterolaemia and coronary risk factors among patients with angiogram-proven premature coronary artery disease in an Asian cohort[J]. PLoS One, 2022, 17(9): e0273896. doi: 10.1371/journal.pone.0273896
[9] Nordestgaard BG, Chapman MJ, Humphries SE, et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society[J]. Eur Heart J, 2013, 34(45): 3478-3490. doi: 10.1093/eurheartj/eht273
[10] Chen B, Xie F, Tang C, et al. Study of five pubertal transition-related gene polymorphisms as risk factors for premature coronary artery disease in a Chinese Han population[J]. PLoS One, 2015, 10(8): e0136496. doi: 10.1371/journal.pone.0136496
[11] Li S, Zhang Y, Zhu CG, et al. Identification of familial hypercholesterolemia in patients with myocardial infarction: A Chinese cohort study[J]. J Clin Lipidol, 2016, 10(6): 1344-1352. doi: 10.1016/j.jacl.2016.08.013
[12] Bernelot Moens SJ, Verweij SL, Schnitzler JG, et al. Remnant cholesterol elicits arterial wall inflammation and a multilevel cellular immune response in Humans[J]. Arterioscler Thromb Vasc Biol, 2017, 37(5): 969-975. doi: 10.1161/ATVBAHA.116.308834
[13] 中国中西医结合学会检验医学专业委员会. 非传统血脂指标与动脉粥样硬化性心血管疾病风险管理中国专家共识[J]. 中华预防医学杂志, 2022, 20(4): 405-421.
[14] Jørgensen AB, Frikke-Schmidt R, West AS, et al. Genetically elevated non-fasting triglycerides and calculated remnant cholesterol as causal risk factors for myocardial infarction[J]. Eur Heart J, 2013, 34(24): 1826-1833. doi: 10.1093/eurheartj/ehs431
[15] Holmes MV, Millwood IY, Kartsonaki C, et al. Lipids, lipoproteins, and metabolites and risk of myocardial infarction and stroke[J]. J Am Coll Cardiol, 2018, 71(6): 620-632. doi: 10.1016/j.jacc.2017.12.006
[16] Varbo A, Benn M, Nordestgaard BG. Remnant cholesterol as a cause of ischemic heart disease: evidence, definition, measurement, atherogenicity, high risk patients, and present and future treatment[J]. Pharmacol Ther, 2014, 141(3): 358-367. doi: 10.1016/j.pharmthera.2013.11.008
[17] Varbo A, Nordestgaard BG. Directly measured vs. calculated remnant cholesterol identifies additional overlooked individuals in the general population at higher risk of myocardial infarction[J]. Eur Heart J, 2021, 42(47): 4833-4843. doi: 10.1093/eurheartj/ehab293
[18] Sandesara PB, Virani SS, Fazio S, et al. The forgotten lipids: triglycerides, remnant cholesterol, and atherosclerotic cardiovascular disease risk[J]. Endocr Rev, 2019, 40(2): 537-557. doi: 10.1210/er.2018-00184
[19] Salinas C, Chapman MJ. Remnant lipoproteins: are they equal to or more atherogenic than LDL?[J]. Curr Opin Lipidol, 2020, 31(3): 132-139. doi: 10.1097/MOL.0000000000000682
[20] Wadström BN, Wulff AB, Pedersen KM, et al. Elevated remnant cholesterol increases the risk of peripheral artery disease, myocardial infarction, and ischaemic stroke: a cohort-based study[J]. Eur Heart J, 2022, 43(34): 3258-3269. doi: 10.1093/eurheartj/ehab705
[21] McNamara JR, Shah PK, Nakajima K, et al. Remnant-like particle(RLP)cholesterol is an independent cardiovascular disease risk factor in women: results from the Framingham Heart Study[J]. Atherosclerosis, 2001, 154(1): 229-236. doi: 10.1016/S0021-9150(00)00484-6
[22] Varbo A, Freiberg JJ, Nordestgaard BG. Extreme nonfasting remnant cholesterol vs extreme LDL cholesterol as contributors to cardiovascular disease and all-cause mortality in 90000 individuals from the general population[J]. Clin Chem, 2015, 61(3): 533-543. doi: 10.1373/clinchem.2014.234146
[23] Song Y, Zhao Y, Bai X, et al. Remnant cholesterol is independently asssociated with an increased risk of peripheral artery disease in type 2 diabetic patients[J]. Front Endocrinol(Lausanne), 2023, 14: 1111152. doi: 10.3389/fendo.2023.1111152
[24] Wadström BN, Wulff AB, Pedersen KM, et al. Elevated remnant cholesterol increases the risk of peripheral artery disease, myocardial infarction, and ischaemic stroke: a cohort-based study[J]. Eur Heart J, 2022, 43(34): 3258-3269. doi: 10.1093/eurheartj/ehab705
[25] Wang K, Wang R, Yang J, et al. Remnant cholesterol and atherosclerotic cardiovascular disease: Metabolism, mechanism, evidence, and treatment[J]. Front Cardiovasc Med, 2022, 9: 913869. doi: 10.3389/fcvm.2022.913869
[26] Nordestgaard BG. Triglyceride-Rich Lipoproteins and Atherosclerotic Cardiovascular Disease: New Insights From Epidemiology, Genetics, and Biology[J]. Circ Res, 2016, 118(4): 547-563. doi: 10.1161/CIRCRESAHA.115.306249
[27] Sandesara PB, Virani SS, Fazio S, et al. The forgotten lipids: triglycerides, remnant cholesterol, and atherosclerotic cardiovascular disease risk[J]. Endocr Rev, 2019, 40(2): 537-557. doi: 10.1210/er.2018-00184
[28] Miller YI, Choi SH, Fang L, et al. Lipoprotein modification and macrophage uptake: role of pathologic cholesterol transport in atherogenesis[J]. Subcell Biochem, 2010, 51: 229-251.
[29] Rosenson RS, Davidson MH, Hirsh BJ, et al. Genetics and causality of triglyceride-rich lipoproteins in atherosclerotic cardiovascular disease[J]. J Am Coll Cardiol, 2014, 64(23): 2525-2540. doi: 10.1016/j.jacc.2014.09.042
[30] Wang A, Tian X, Zuo Y, et al. Age dependent association between remnant cholesterol and cardiovascular disease[J]. Atheroscler Plus, 2021, 45: 18-24. doi: 10.1016/j.athplu.2021.09.004
[31] Baratta F, Cocomello N, Coronati M, et al. Cholesterol Remnants, Triglyceride-Rich Lipoproteins and Cardiovascular Risk[J]. Int J Mol Sci, 2023, 24(5): 4268. doi: 10.3390/ijms24054268
[32] Shin HK, Kim YK, Kim KY, et al. Remnant lipoprotein particles induce apoptosis in endothelial cells by NAD(P)H oxidase-mediated production of superoxide and cytokines via lectin-like oxidized low-density lipoprotein receptor-1 activation: prevention by cilostazol[J]. Circulation, 2004, 109(8): 1022-1028. doi: 10.1161/01.CIR.0000117403.64398.53
[33] Chen Y, Li G, Guo X, et al. The effects of calculated remnant-like particle cholesterol on incident cardiovascular disease: insights from a general chinese population[J]. J Clin Med, 2021, 10(15): 3388. doi: 10.3390/jcm10153388
[34] Varbo A, Benn M, Tybjærg-Hansen A, et al. Elevated remnant cholesterol causes both low-grade inflammation and ischemic heart disease, whereas elevated low-density lipoprotein cholesterol causes ischemic heart disease without inflammation[J]. Circulation, 2013, 128(12): 1298-309. doi: 10.1161/CIRCULATIONAHA.113.003008
[35] Gaggini M, Gorini F, Vassalle C. Lipids in atherosclerosis: pathophysiology and the role of calculated lipid indices in assessing cardiovascular risk in patients with hyperlipidemia[J]. Int J Mol Sci, 2022, 24(1): 75. doi: 10.3390/ijms24010075
[36] Liu HH, Li S, Cao YX, et al. Association of triglyceride-rich lipoprotein-cholesterol with recurrent cardiovascular events in statin-treated patients according to different inflammatory status[J]. Atherosclerosis, 2021, 330: 29-35. doi: 10.1016/j.atherosclerosis.2021.06.907
[37] Zhou L, Liu J, An Y, et al. Plasma homocysteine level is independently associated with conventional atherogenic lipid profile and remnant cholesterol in adults[J]. Front Cardiovasc Med, 2022, 9: 898305. doi: 10.3389/fcvm.2022.898305
[38] Tian Y, Wu W, Qin L, et al. Prognostic value of remnant cholesterol in patients with coronary heart disease: A systematic review and meta-analysis of cohort studies[J]. Front Cardiovasc Med, 2022, 9: 951523.
-




 下载:
下载: