Comparison of cardiac output measured by thermodilution and indirect Fick methods in patients with pulmonary hypertension due to left heart disease
-
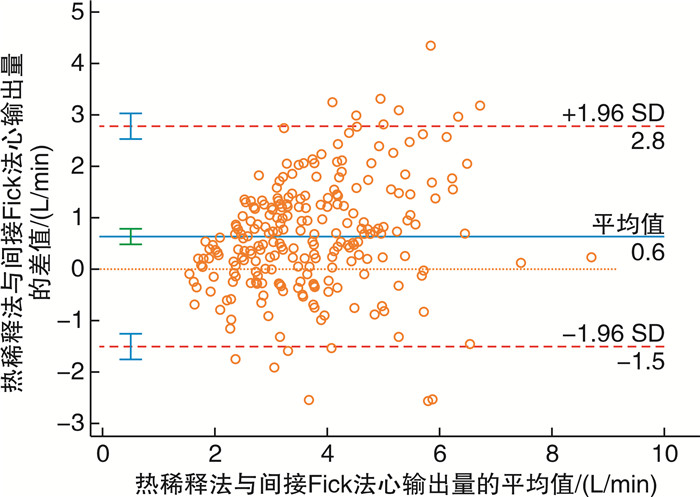
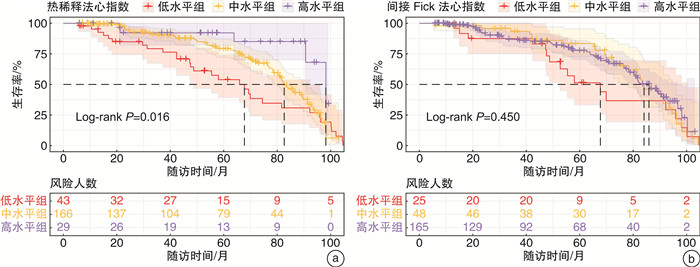
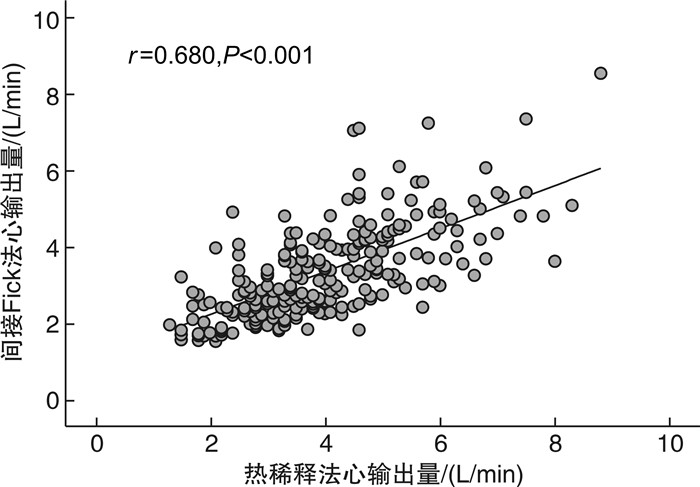
摘要: 目的 比较热稀释法与间接Fick法测量的心输出量在左心疾病相关肺高血压(PH-LHD)患者中的差异,探讨两种方法测定的心指数与患者预后的关系。方法 连续收集2013年9月—2021年12月在南京医科大学第一附属医院心内科经右心导管检查确诊PH-LHD患者的临床资料及随访数据。随访截至2022年4月,主要终点事件为全因死亡。采用Pearson相关系数及Bland-Altman散点图评估两种心输出量测量方法的相关性与一致性。采用X-tile软件计算心指数的最佳临界值。使用Kaplan-Meier生存曲线及Cox回归模型评价热稀释法与间接Fick法测定的心指数在PH-LHD患者中的预后价值。结果 纳入分析的238例PH-LHD患者,热稀释法测定的心输出量为(4.03±1.46) L/min,间接Fick法测定的心输出量为(3.39±1.20) L/min,两者比较差异有统计学意义(P < 0.001)。热稀释法与间接Fick法心输出量呈中度正相关(r=0.680,P < 0.001)。两者心输出量的差值均值为0.64 L/min,一致性界限为-1.50~2.78 L/min,百分比误差>20%的比例为53.4%,心输出量差值>1 L/min的比例为38.2%。根据X-tile软件计算的最佳临界值将热稀释法及间接Fick法心指数分为低、中、高水平3组。Kaplan-Meier曲线显示,热稀释法心指数的组间生存率比较差异有统计学意义(log-rank P=0.016);间接Fick法心指数的组间生存率比较差异无统计学意义(log-rank P=0.450)。Cox回归模型中,根据年龄、性别进行校正,热稀释法心指数每增加1 L/min/m2,患者的死亡风险降低25%(P=0.038),中水平组、低水平组的死亡风险相比于高水平组分别增加了169%(P=0.034)及252%(P=0.011);进一步校正多重混杂因素后,热稀释法心指数不具有独立的预测价值。间接Fick法心指数作为连续变量及分组变量均与患者的全因死亡无关。结论 热稀释法与间接Fick法心输出量在PH-LHD患者中呈中度正相关,但一致性欠佳。热稀释法心指数对PH-LHD患者预后有一定预测价值,而间接Fick法心指数的预后价值并不理想。
-
关键词:
- 左心疾病相关肺高血压 /
- 心输出量 /
- 热稀释法 /
- 间接Fick法 /
- 全因死亡
Abstract: Objective To compare the difference in cardiac output measured by thermodilution and indirect Fick methods in patients with pulmonary hypertension due to left heart disease(PH-LHD), and to investigate the relationship between the calculated cardiac index and prognosis.Methods Clinical data and follow-up information were collected from PH-LHD patients diagnosed by right heart catheterization in the Department of Cardiology, the First Affiliated Hospital with Nanjing Medical University from September 2013 to December 2021. Patients were followed up until April 2022, with the primary endpoint of all-cause mortality. Pearson correlation coefficients and Bland-Altman scatter plots were used to evaluate the correlation and consistency of the two measurement methods. The optimal cutoff values for the cardiac index categories were determined using the X-tile software. Kaplan-Meier curves and Cox regression models were used to evaluate the prognostic value of the cardiac index determined by the thermodilution and indirect Fick methods in PH-LHD patients. Results A total of 238 PH-LHD patients were included. The cardiac output measured by thermodilution was(4.03±1.46) L/min, and that measured by indirect Fick was(3.39±1.20) L/min, with a statistically significant difference(P < 0.001). The cardiac outputs measured by thermodilution and indirect Fick had a moderately positive correlation(r=0.680, P < 0.001). The mean difference in cardiac output between the two methods was 0.64 L/min, with a consistency limit of-1.50-2.78 L/min, and a percentage error>20% of 53.4%. The proportion of patients with a difference in cardiac output>1 L/min was 38.2%. Based on the optimal critical values calculated by X-tile, the Kaplan-Meier curve showed that there was a statistically significant difference in the survival rate between the thermodilution cardiac index groups(log-rank P=0.016), but not in the indirect Fick cardiac index groups(log-rank P=0.450). After adjusting for age and sex, for each 1 L/min/m2increase in the thermodilution cardiac index, the risk of death decreased by 25%(P=0.038), and the risk of death in the middle and low cardiac index groups increased by 169%(P=0.034) and 252%(P=0.011), respectively, compared with the high cardiac index group. After further adjusting for multiple confounding factors, however, both the thermodilution and indirect Fick-measured cardiac index, as a continuous or categorical variable, did not show a significant association with all-cause mortality.Conclusion There is a moderate positive correlation between the cardiac outputs measured by thermodilution and indirect Fick methods in PH-LHD patients, but the consistency is poor. The thermodilution cardiac index demonstrates a certain predictive value for the prognosis of PH-LHD patients, while the prognostic value of the indirect Fick cardiac index is not ideal. -

-
表 1 死亡组与存活组基线数据比较
Table 1. Baseline data in the death group and survival group
例(%), X±S, M(P25, P75) 项目 死亡组(100例) 存活组(138例) P值 年龄/岁 59.0±10.5 52.3±12.9 < 0.001 性别 0.480 女 34(45.3) 41(54.7) 男 66(40.5) 97(59.5) BMI/(kg/m2) 23.6±3.7 25.0±4.6 0.013 体表面积/m2 1.7±0.2 1.8±0.2 0.013 NT-pro BNP/(ng/L) 2 487 (1 178,5 844) 1 630 (806,3 432) 0.003 外周SaO2/% 96.1±3.2 97.2±2.1 0.002 血红蛋白/(g/L) 134.9±19.5 137.8±19.6 0.258 超声心动图数据 左心室射血分数/% 37.1±15.4 35.4±14.3 0.397 肺动脉收缩压/mmHg 50.8±17.1 47.0±16.3 0.106 右心导管数据 上腔静脉压/mmHg 14.9±7.5 13.0±5.7 0.035 上腔静脉SPO2/% 55.1±10.3 63.0±10.6 < 0.001 右心房平均压/mmHg 10.6±6.1 8.8±4.8 0.016 PAWP/mmHg 23.5±5.0 21.9±5.1 0.015 肺动脉收缩压/mmHg 57.6±15.9 49.4±14.0 < 0.001 肺动脉舒张压/mmHg 27.2±9.3 24.2±6.7 0.008 mPAP/mmHg 39.2±10.5 34.4±8.6 < 0.001 肺动脉SPO2/% 52.6±11.6 61.5±10.6 < 0.001 PVR/Wood 5.3±3.2 3.2±2.3 < 0.001 热稀释法心输出量/(L/min) 3.5±1.2 4.4±1.5 < 0.001 间接Fick法心输出量/(L/min) 3.0±1.1 3.7±1.2 < 0.001 热稀释法心指数/(L/min/m2) 2.0±0.7 2.5±0.8 < 0.001 间接Fick法心指数/(L/min/m2) 1.7±0.6 2.1±0.6 < 0.001 表 2 心指数与全因死亡的Cox比例风险模型
Table 2. The Cox proportional hazards model of cardiac index and all-cause mortality
变量 未调整模型HR(95%CI) 模型1HR(95%CI) 模型2HR(95%CI) 热稀释法心指数 每增加1 L/min/m2 0.75(0.57~0.98)1) 0.75(0.58~0.99)1) 0.95(0.67~1.35) 高水平组 参考 参考 参考 中水平组 2.62(1.05~6.50)1) 2.69(1.08~6.73)1) 1.73(0.67~4.45) 低水平组 3.76(1.44~9.82)1) 3.52(1.33~9.31)1) 1.76(0.61~5.11) 间接Fick法心指数 每增加1 L/min/m2 1.01(0.71~1.43) 1.00(0.70~1.42) 1.42(0.98~2.05) 高水平组 参考 参考 参考 中水平组 1.08(0.68~1.70) 1.02(0.65~1.62) 0.68(0.39~1.12) 低水平组 1.45(0.82~2.56) 1.49(0.84~2.65) 0.74(0.34~1.61) 模型1:校正了年龄、性别;模型2:校正了模型1+log2NT-proBNP,右心导管测量的平均右心房压、肺动脉收缩压、mPAP、PAWP。1)P < 0.05。 -
[1] 唐愿, 张海锋, 李新立. 左心疾病相关肺高血压的诊治进展[J]. 中华心血管病杂志, 2022, 50(1): 8-13. https://www.cnki.com.cn/Article/CJFDTOTAL-NKYT202206012.htm
[2] 中华医学会心血管病学分会肺血管病学组, 中华心血管病杂志编辑委员会. 中国肺高血压诊断和治疗指南2018[J]. 中华心血管病杂志, 2018, 46(12): 933-964. doi: 10.3760/cma.j.issn.0253-3758.2018.12.006
[3] Rosenkranz S, Gibbs JSR, Wachter R, et al. Left ventricular heart failure and pulmonary hypertension[J]. Eur Heart J, 2016, 37(12): 942-954. doi: 10.1093/eurheartj/ehv512
[4] 王璐瑶, 闫旭龙, 杨晓敏, 等. 左心疾病相关性肺动脉高压的临床特点分析[J]. 临床心血管病杂志, 2021, 37(5): 462-467. https://lcxxg.whuhzzs.com/article/doi/10.13201/j.issn.1001-1439.2021.05.014
[5] Hoeper MM, Maier R, Tongers J, et al. Determination of cardiac output by the Fick method, thermodilution, and acetylene rebreathing in pulmonary hypertension[J]. Am J Respir Crit Care Med, 1999, 160(2): 535-541. doi: 10.1164/ajrccm.160.2.9811062
[6] Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension[J]. Eur Heart J, 2022, 43(38): 3618-3731. doi: 10.1093/eurheartj/ehac237
[7] Axler O, Tousignant C, Thompson CR, et al. Comparison of transesophageal echocardiographic, fick, and thermodilution cardiac output in critically ill patients[J]. J Crit Care, 1996, 11(3): 109-116. doi: 10.1016/S0883-9441(96)90006-4
[8] Dhingra VK, Fenwick JC, Walley KR, et al. Lack of agreement between thermodilution and fick cardiac output in critically ill patients[J]. Chest, 2002, 122(3): 990-997. doi: 10.1378/chest.122.3.990
[9] Gonzalez J, Delafosse C, Fartoukh M, et al. Comparison of bedside measurement of cardiac output with the thermodilution method and the Fick method in mechanically ventilated patients[J]. Crit Care, 2003, 7(2): 171-178. doi: 10.1186/cc1848
[10] Tehrani DM, Grinstein J, Kalantari S, et al. Cardiac Output Assessment in Patients Supported with Left Ventricular Assist Device: Discordance Between Thermodilution and Indirect Fick Cardiac Output Measurements[J]. ASAIO J, 2017, 63(4): 433-437. doi: 10.1097/MAT.0000000000000528
[11] Azih NI, Read JM, Jackson GR, et al. Cardiac output assessment methods in left ventricular assist device patients: A problem of heteroscedasticity[J]. J Heart Lung Transplant, 2023, 42(2): 145-149. doi: 10.1016/j.healun.2022.10.021
[12] Alkhodair A, Tsang MYC, Cairns JA, et al. Comparison of thermodilution and indirect Fick cardiac outputs in pulmonary hypertension[J]. Int J Cardiol, 2018, 258: 228-231. doi: 10.1016/j.ijcard.2018.01.076
[13] Fares WH, Blanchard SK, Stouffer GA, et al. Thermodilution and Fick cardiac outputs differ: impact on pulmonary hypertension evaluation[J]. Can Respir J, 2012, 19(4): 261-266. doi: 10.1155/2012/261793
[14] 朱锋, 熊长明, 何建国, 等. 间歇热稀释法与间接Fick法测定肺动脉高压患者心排出量结果的比较[J]. 中国循环杂志, 2011, 26(4): 248-251. doi: 10.3969/j.issn.1000-3614.2011.04.004
[15] Desole S, Obst A, Habedank D, et al. Comparison between thermodilution and Fick methods for resting and exercise-induced cardiac output measurement in patients with chronic dyspnea[J]. Pulm Circ, 2022, 12(3): e12128. doi: 10.1002/pul2.12128
[16] Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization[J]. Clin Cancer Res, 2004, 10(21): 7252-7259. doi: 10.1158/1078-0432.CCR-04-0713
[17] Narang N, Thibodeau JT, Parker WF, et al. Comparison of Accuracy of Estimation of Cardiac Output by Thermodilution Versus the Fick MethodsUsing Measured Oxygen Uptake[J]. Am J Cardiol, 2022, 176: 58-65. doi: 10.1016/j.amjcard.2022.04.026
[18] Renner LE, Morton MJ, Sakuma GY. Indicator amount, temperature, and intrinsic cardiac output affect thermodilution cardiac output accuracy and reproducibility[J]. Crit Care Med, 1993, 21(4): 586-597. doi: 10.1097/00003246-199304000-00021
[19] Van Grondelle A, Ditchey RV, Groves BM, et al. Thermodilution method overestimates low cardiac output in humans[J]. Am J Physiol, 1983, 245(4): H690-H692.
[20] Cigarroa RG, Lange RA, Williams RH, et al. Underestimation of cardiac output by thermodilution in patients with tricuspid regurgitation[J]. Am J Med, 1989, 86(4): 417-420. doi: 10.1016/0002-9343(89)90339-2
[21] Opotowsky AR, Hess E, Maron BA, et al. Thermodilution vs Estimated Fick Cardiac Output Measurement in Clinical Practice: An Analysis of Mortality From the Veterans Affairs Clinical Assessment, Reporting, and Tracking(VA CART)Program and Vanderbilt University[J]. JAMA Cardiol, 2017, 2(10): 1090-1099. doi: 10.1001/jamacardio.2017.2945
[22] Chase PJ, Davis PG, Wideman L, et al. Comparison of Estimations Versus Measured Oxygen Consumption at Rest in Patients With Heart Failure and Reduced Ejection Fraction Who Underwent Right-Sided Heart Catheterization[J]. Am J Cardiol, 2015, 116(11): 1724-1730. doi: 10.1016/j.amjcard.2015.08.051
[23] Fakler U, Pauli C, Hennig M, et al. Assumed oxygen consumption frequently results in large errors in the determination of cardiac output[J]. J Thorac Cardiovasc Surg, 2005, 130(2): 272-276. doi: 10.1016/j.jtcvs.2005.02.048
[24] Grafton G, Cascino TM, Perry D, et al. Resting Oxygen Consumption and Heart Failure: Importance of Measurement for Determination of Cardiac Output With the Use of the Fick Principle[J]. J Card Fail, 2020, 26(8): 664-672. doi: 10.1016/j.cardfail.2019.02.004
[25] Narang N, Thibodeau JT, Levine BD, et al. Inaccuracy of estimated resting oxygen uptake in the clinical setting[J]. Circulation, 2014, 129(2): 203-210. doi: 10.1161/CIRCULATIONAHA.113.003334
[26] Kresoja KP, Faragli A, Abawi D, et al. Thermodilution vs estimated Fick cardiac output measurement in an elderly cohort of patients: A single-centre experience[J]. PLoS One, 2019, 14(12): e0226561. doi: 10.1371/journal.pone.0226561
[27] Vanderpool RR, Saul M, Nouraie M, et al. Association Between Hemodynamic Markers of Pulmonary Hypertension and Outcomes in Heart Failure With Preserved Ejection Fraction[J]. JAMA Cardiol, 2018, 3(4): 298-306. doi: 10.1001/jamacardio.2018.0128
[28] Sugimoto K, Yoshihisa A, Nakazato K, et al. Significance of Pulmonary Vascular Resistance and Diastolic Pressure Gradient on the New Definition of Combined Post-Capillary Pulmonary Hypertension[J]. Int Heart J, 2020, 61(2): 301-307. doi: 10.1536/ihj.19-476
[29] Caravita S, Faini A, Carolino D'araujo S, et al. Clinical phenotypes and outcomes of pulmonary hypertension due to left heart disease: Role of the pre-capillary component[J]. PLoS One, 2018, 13(6): e0199164. doi: 10.1371/journal.pone.0199164
-




 下载:
下载: