The distribution of circulating T follicular helper cells and its association with Gensini score in patients with acute coronary syndrome
-
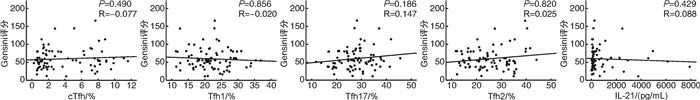
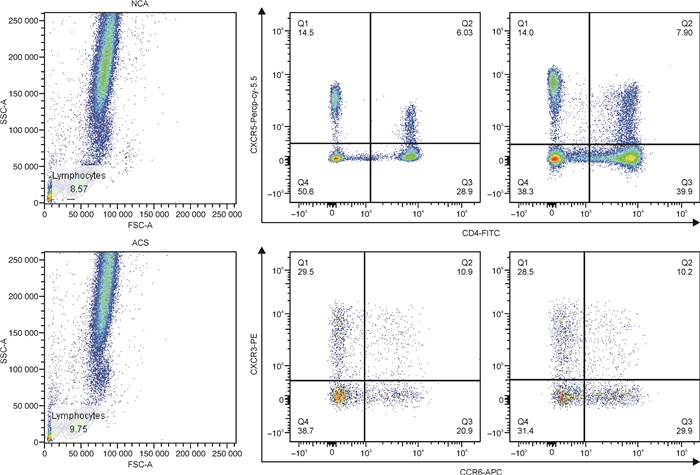
摘要: 目的 探究外周血循环滤泡辅助T细胞(circulating T follicular helper,cTfh)及其亚型在急性冠状动脉综合征(ACS)患者中的分布情况,及其与冠状动脉(冠脉)狭窄程度(Gensini评分)的关系。方法 采用酶联免疫吸附法检测ACS患者(ACS组,85例)及冠脉造影正常者(NCA组,43例)外周血白细胞介素21(interleukin-21,IL-21)水平,应用流式细胞术分析两组试验对象外周血cTfh水平及其亚型所占比例,同时进行相关性分析、回归分析探究其与冠心病传统危险因素及Gensini评分的关系。结果 与NCA组相比,ACS患者外周血IL-21及cTfh水平显著升高(IL-21:74.6 pg/mL vs 153.6 pg/mL,P < 0.001;cTfh:1.40% vs 2.23%,P=0.016),且cTfh亚型比例失调(以Tfh17亚型升高为主:24.0% vs 27.2 %,P=0.015)。冠心病传统危险因素中,仅低密度脂蛋白胆固醇水平与Tfh2亚型呈弱正相关性(r=0.213,P=0.016);高水平IL-21(OR=4.657,95%CI:2.053~10.563,P < 0.001)、cTfh(OR=1.250,95%CI:1.063~1.470,P < 0.007)及Tfh17(OR=1.063,95%CI:1.002~1.129,P < 0.044)为ACS的危险因素,但均与Gensini评分无关联。结论 ACS患者外周血IL-21及cTfh水平升高,cTfh亚型失调。Tfh可能参与ACS的发生发展,但尚未发现其与冠脉狭窄程度的关系。Abstract: Objective To investigate the distribution of circulating T follicular helper cells (cTfh) and its association with the Gensini score in patients with acute coronary syndrome (ACS).Methods The cTfh and its subsets from a total of 85 ACS patients (ACS group) and 43 patients with normal coronary arteries (NCA group) were investigated using flow cytometry. Plasma level of interleukin-21 (IL-21) were examined and the Gensini score was calculated from coronary angiograms. The association of IL-21/cTfh with atherosclerosis risk factors or Gensini score was assessed by correlation analysis and regression analysis.Results The percentage of total cTfh cells and Tfh17 cells in cTfh cells were significantly increased in the ACS group compared with the NCA group(cTfh: 1.40% vs 2.23%, P=0.016; Tfh17: 24.0% vs 27.2 %, P=0.015). In addition, IL-21 content in plasma increased in the ACS group(74.6 pg/mL vs 153.6 pg/mL, P < 0.001). Only the percentage of Tfh2 in cTfh was related to low density lipoprotein cholesterol LDL-C level(r=0.213, P=0.016). Higher plasma IL-21 concentration(OR=4.657, 95%CI: 2.053-10.563, P < 0.001), cTfh(OR=1.250, 95%CI: 1.063-1.470, P < 0.007), and Tfh17(OR=1.063, 95%CI: 1.002-1.129, P < 0.044) were risk factors of ACS, however, none of them were associated with the Gensini score.Conclusion Patients with ACS show a high expression of IL-21 and cTfh, as well as a dysregulation of cTfh subtypes. Tfh may be involved in the occurrence and development of ACS, but the relationship between cTfh and the degree of coronary artery stenosis has not been found yet.
-

-
表 1 研究人群基本临床资料
Table 1. Baseline clinical data
例(%), X±S, M(P25, P25) 项目 NCA组(43例) ACS组(85例) P 年龄/岁 56.27±9.37 58.23±8.56 0.237 男性 32(74.4) 66(77.6) 0.684 高血压 23(53.5) 48(56.5) 0.748 糖尿病/糖耐量异常 8(18.6) 17(20.0) 0.851 血脂异常 5(11.6) 2(2.4) 0.042 吸烟 18(41.9) 49(57.6) 0.091 心血管家族史 11(25.6) 12(14.1) 0.111 收缩压/mmHg 135.0±14.6 132.2±15.0 0.328 舒张压/mmHg 78.8±11.2 79.5±13.6 0.748 心率/(次/min) 79.0±14.5 74.0±13.0 0.052 射血分数/% 61.7(58.4,64.0) 59.5(48.8,64.0) 0.218 肌钙蛋白T/(ng/mL) 0.026(0.005,4.400) 14.500(0.860,22.150) < 0.001 总胆固醇/(mg/dL) 4.06±0.79 3.76±0.84 0.056 甘油三酯/(mmol/L) 1.47(1.02,2.02) 1.30(0.89,1.81) 0.141 低密度脂蛋白胆固醇/(mg/dL) 2.38±0.66 2.15±0.68 0.074 高密度脂蛋白胆固醇/(mg/dL) 0.87(0.74,1.17) 0.96(0.80,1.12) 0.563 肌酐/(μmol/L) 69.2(57.9,80.5) 70.7(63.0,80.0) 0.679 尿酸/(μmol/L) 293.6(249.3,336.9) 302.0(277.6,352.3) 0.395 空腹血糖/(mmol/L) 5.8(4.9,7.0) 5.9(5.1,8.0) 0.326 糖化血红蛋白/% 5.5(5.4,5.7) 5.6(5.2,6.0) 0.231 表 2 NCA组和ACS组cTfh细胞和血浆IL-21水平
Table 2. Levels of cTfh cells and IL-21
M(P25, P25) 项目 NCA组(43例) ACS组(85例) P IL-21/(pg/mL) 74.6(61.5,119.1) 153.6(87.9,627.7) < 0.001 cTfh/% 1.40(0.99,2.63) 2.23(1.18,7.19) 0.016 Tfh1/% 21.7(19.8,26.7) 21.3(18.1,25.6) 0.386 Tfh2/% 24.6(18.8,41.0) 24.3(17.6,32.3) 0.718 Tfh17/% 24.0(19.8,28.4) 27.2(22.6,31.9) 0.015 表 3 ACS患者cTfh、IL-21与AS危险因素的相关性分析
Table 3. Correlation between cTfh, IL-21 and risk factors of AS in patients with ACS
变量 IL-21 cTfh Tfh1 Tfh2 Tfh17 r P r P r P r P r P 年龄 0.112 0.208 0.015 0.865 0.092 0.303 -0.137 0.124 0.087 0.330 收缩压 0.077 0.388 0.054 0.547 0.044 0.620 0.156 0.079 -0.036 0.689 舒张压 0.028 0.749 0.088 0.325 -0.081 0.365 0.074 0.408 0.118 0.183 糖化血红蛋白 -0.049 0.584 -0.017 0.846 -0.124 0.165 -0.140 0.116 -0.136 0.125 甘油三酯 -0.035 0.699 0.161 0.069 -0.089 0.320 0.061 0.494 -0.043 0.629 低密度脂蛋白胆固醇 -0.015 0.871 -0.033 0.709 0.028 0.757 0.213 0.016 0.107 0.230 表 4 ACS患者Gensini评分的logistic回归分析
Table 4. Logistic regression analysis of cTfh, IL-21 and Gensini score in ACS patients
变量 未调整 经调整 OR(95%CI) P OR(95%CI) P IL-21 0.573(0.233~1.408) 0.902 0.536(0.213~1.347) 0.185 cTfh 0.980(0.867~1.120) 0.763 0.983(0.858~1.126) 0.803 Tfh1 1.000(0.928~1.078) 0.998 0.999(0.926~1.079) 0.989 Tfh2 1.002(0.950~1.057) 0.935 1.009(0.956~1.066) 0.739 Tfh17 1.061(0.991~1.135) 0.087 1.048(0.977~1.123) 0.194 调整因素:年龄和性别。 表 5 胸痛者患ACS的logistic回归分析
Table 5. Logistic regression analysis of cTfh, IL-21 and ACS in patients with chest pain
变量 未调整 经调整 OR(95%CI) P OR(95%CI) P IL-21 4.818(2.136~10.869) < 0.001 4.657(2.053~10.563) < 0.001 cTfh 1.254(1.067~1.473) 0.006 1.250(1.063~1.470) 0.007 Tfh1 0.978(0.914~1.045) 0.505 0.976(0.911~1.045) 0.478 Tfh2 1.005(0.963~1.049) 0.809 1.008(0.965~1.053) 0.733 Tfh17 1.067(1.005~1.132) 0.033 1.063(1.002~1.129) 0.044 调整因素:年龄和性别。 -
[1] Nichols M, Townsend N, Scarborough P, et al. Cardiovascular disease in Europe 2014: epidemiological update[J]. Eur Heart J, 2014, 35(42): 2950-2959. doi: 10.1093/eurheartj/ehu299
[2] Wolf D, Ley K. Immunity and inflammation in atherosclerosis[J]. Circ Res, 2019, 124(2): 315-327. doi: 10.1161/CIRCRESAHA.118.313591
[3] 陈荣伴, 邹琪. CD4+CD25+调节性T细胞对慢性淋巴细胞白血病预后的影响[J]. 临床血液学杂志, 2015, 28(2): 221-223.
[4] Kumar V, Prabhu SD, Bansal SS, et al. CD4+ T-lymphocytes exhibit biphasic kinetics post-myocardial infarction[J]. Front Cardiovasc Med, 2022, 9: 992653. doi: 10.3389/fcvm.2022.992653
[5] Saigusa R, Winkels H, Ley K. T cell subsets and functions in atherosclerosis[J]. Nat Rev Cardiol, 2020, 17(7): 387-401. doi: 10.1038/s41569-020-0352-5
[6] Walker L. The link between circulating follicular helper T cells and autoimmunity[J]. Nat Rev Immunol, 2022, 22(9): 567-575. doi: 10.1038/s41577-022-00693-5
[7] Wei X, Niu X. T follicular helper cells in autoimmune diseases[J]. J Autoimmun, 2023, 134: 102976. doi: 10.1016/j.jaut.2022.102976
[8] Vinuesa CG, Linterman MA, Yu D, et al. Follicular Helper T Cells[J]. Annu Rev Immunol, 2016, 34: 335-368. doi: 10.1146/annurev-immunol-041015-055605
[9] Spolski R, Leonard WJ. Interleukin-21: a double-edged sword with therapeutic potential[J]. Nat Rev Drug Discov, 2014, 13(5): 379-395. doi: 10.1038/nrd4296
[10] Kurata I, Matsumoto I, Sumida T. T follicular helper cell subsets: a potential key player in autoimmunity[J]. Immunol Med, 2021, 44(1): 1-9. doi: 10.1080/25785826.2020.1776079
[11] Schmitt N, Bentebibel SE, Ueno H. Phenotype and functions of memory Tfh cells in human blood[J]. Trends Immunol, 2014, 35(9): 436-442. doi: 10.1016/j.it.2014.06.002
[12] Iwamoto Y, Ueno H. Circulating T Follicular Helper Subsets in Human Blood[J]. Methods Mol Biol, 2022, 2380: 29-39.
[13] Mintz MA, Cyster JG. T follicular helper cells in germinal center B cell selection and lymphomagenesis[J]. Immunol Rev, 2020, 296(1): 48-61. doi: 10.1111/imr.12860
[14] Geng J, Wei H, Shi B, et al. Bach2 Negatively Regulates T Follicular Helper Cell Differentiation and Is Critical for CD4+ T Cell Memory[J]. J Immunol, 2019, 202(10): 2991-2998. doi: 10.4049/jimmunol.1801626
[15] 刘善凤, 胡丽华. SLE患者调节性B淋巴细胞的检测和临床意义[J]. 临床血液学杂志, 2015, 28(12): 1025-1027.
[16] Xu T, Yan T, Li F, et al. The Role of Different Circulating T Follicular Helper Cell Markers in Rheumatoid Arthritis[J]. J Interferon Cytokine Res, 2022, 42(3): 108-117. doi: 10.1089/jir.2021.0168
[17] Bemani P, Eklund KK, Ali-Hassanzadeh M, et al. Proportion of T follicular helper cells in peripheral blood of rheumatoid arthritis patients: a systematic review and meta-analysis[J]. Expert Rev Clin Immunol, 2021, 17(6): 667-680. doi: 10.1080/1744666X.2021.1915770
[18] Chen W, Yang F, Xu G, et al. Follicular helper T cells and follicular regulatory T cells in the immunopathology of primary Sjögren's syndrome[J]. J Leukoc Biol, 2021, 109(2): 437-447. doi: 10.1002/JLB.5MR1020-057RR
[19] Xiao F, Zhang HY, Liu YJ, et al. Higher frequency of peripheral blood interleukin 21 positive follicular helper T cells in patients with ankylosing spondylitis[J]. J Rheumatol, 2013, 40(12): 2029-2037. doi: 10.3899/jrheum.130125
[20] Jin X, Chen J, Wu J, et al. Aberrant expansion of follicular helper T cell subsets in patients with systemic lupus erythematosus[J]. Front Immunol, 2022, 13: 928359. doi: 10.3389/fimmu.2022.928359
[21] Basto AP, Graca L. Micro RNAs in Tfh regulation: Small molecules with a big impact[J]. Eur J Immunol, 2021, 51(2): 292-295. doi: 10.1002/eji.202049086
[22] Viisanen T, Ihantola EL, Näntö-Salonen K, et al. Circulating CXCR5+PD-1+ICOS+ Follicular T Helper Cells Are Increased Close to the Diagnosis of Type 1 Diabetes in Children With Multiple Autoantibodies[J]. Diabetes, 2017, 66(2): 437-447. doi: 10.2337/db16-0714
[23] Yu H, Liu B, Wu G, et al. Dysregulation of circulating follicular helper T cells in type 2 diabetic patients with diabetic retinopathy[J]. Immunol Res, 2021, 69(2): 153-161. doi: 10.1007/s12026-021-09182-8
[24] Ding R, Gao W, He Z, et al. Overrepresentation of Th1-and Th17-like Follicular Helper T Cells in Coronary Artery Disease[J]. J Cardiovasc Transl Res, 2015, 8(9): 503-505. doi: 10.1007/s12265-015-9662-0
[25] Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines[J]. J Thorac Cardiovasc Surg, 2016, 152(5): 1243-1275. doi: 10.1016/j.jtcvs.2016.07.044
[26] Kubota A, Suto A, Suga K, et al. Inhibition of Interleukin-21 prolongs the survival through the promotion of wound healing after myocardial infarction[J]. J Mol Cell Cardiol, 2021, 159: 48-61. doi: 10.1016/j.yjmcc.2021.06.006
[27] Li Y, To K, Kanellakis P, et al. CD4+ natural killer T cells potently augment aortic root atherosclerosis by perforin-and granzyme B-dependent cytotoxicity[J]. Circ Res, 2015, 116(2): 245-254. doi: 10.1161/CIRCRESAHA.116.304734
[28] Wang K, Wen S, Jiao J, et al. IL-21 promotes myocardial ischaemia/reperfusion injury through the modulation of neutrophil infiltration[J]. Br J Pharmacol, 2018, 175(8): 1329-1343.
[29] Ding R, Gao W, He Z, et al. Effect of serum interleukin 21 on the development of coronary artery disease[J]. APMIS, 2014, 122(9): 842-847.
[30] Ding R, Gao W, He Z, et al. Circulating CD4+CXCR5+ T cells contribute to proinflammatory responses in multiple ways in coronary artery disease[J]. Int Immunopharmacol, 2017, 52: 318-323.
-




 下载:
下载: