Research progress of ion channels in the formation mechanism of neutrophil extracellular traps in acute myocardial infarction
-
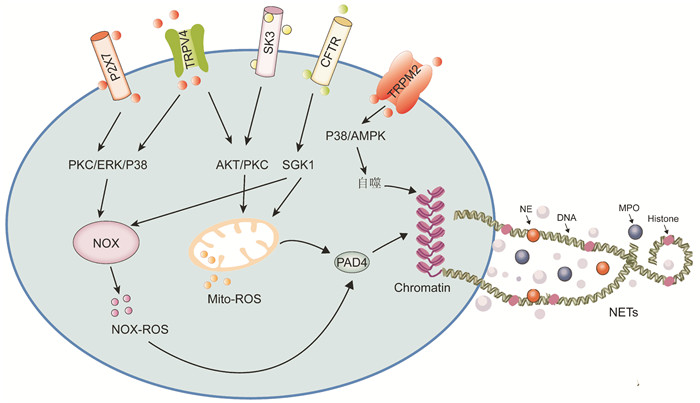
摘要: 中性粒细胞胞外诱捕网(neutrophil extracellular traps,NETs)是由组蛋白和中性粒细胞颗粒蛋白组成的DNA网状结构,在中性粒细胞捕获和杀死病原体过程中起重要作用。在心肌梗死过程中,中性粒细胞激活导致NETs水平的异常升高,加重了炎症反应和组织损伤。目前研究提示,相关离子通道的活化可通过不同途径调控NETs形成,导致心肌梗死后损伤加重、心律失常等心血管不良事件的发生。本文阐述了NETs的形成途径和对急性心肌梗死的作用,并详细描述了中性粒细胞上小电导钙激活钾通道、瞬时感受器电位离子通道、P2X受体和囊性纤维化跨膜电导调节体等在NETs形成中的作用和机制,为减轻心肌梗死后心脏重塑和心律失常提供新的思路。
-
关键词:
- 中性粒细胞胞外诱捕网 /
- 离子通道 /
- 活性氧
Abstract: Neutrophil extracellular traps (NETs) are web-like DNA structures decorated with histones and neutrophil granule proteins in capturing and killing pathogens. During myocardial infarction, an abnormal increase in the level of NETs exacerbates the inflammatory response and tissue damage. The current study suggests that activation of related ion channels may regulate the formation of NETs through different pathways, leading to cardiovascular adverse events such as ventricular remodeling and arrhythmias after myocardial infarction. In this paper, we first described the pathways during NETs formation and their role in acute myocardial infarction. We also summarized in detail the role and mechanism of ion channels in NETs formation during acute myocardial infarction, including SK, TRP, P2X, and CFTR on neutrophils, which may provide new ideas for mitigating cardiac remodeling and arrhythmias after myocardial infarction.-
Key words:
- neutrophil extracellular traps /
- ion channels /
- reactive oxygen species
-

-
[1] Liew PX, Kubes P. The Neutrophil's Role During Health and Disease[J]. Physiol Rev, 2019, 99(2): 1223-1248. doi: 10.1152/physrev.00012.2018
[2] Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria[J]. Science, 2004, 303(5663): 1532-1532. doi: 10.1126/science.1092385
[3] Urban CF, Ermert D, Schmid M, et al. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans[J]. PLoS Pathog, 2009, 5(10): e1000639. doi: 10.1371/journal.ppat.1000639
[4] Sorvillo N, Cherpokova D, Martinod K, et al. Extracellular DNA NET-Works With Dire Consequences for Health[J]. Circ Res, 2019, 125(4): 470-488. doi: 10.1161/CIRCRESAHA.119.314581
[5] 喻珮, 徐承义, 宋丹. 急性心肌梗死后心脏损伤修复的研究进展[J]. 临床心血管病杂志, 2023, 39(7): 558-562. https://www.cnki.com.cn/Article/CJFDTOTAL-LCXB202307013.htm
[6] Eghbalzadeh K, Georgi L, Louis T, et al. Compromised Anti-inflammatory Action of Neutrophil Extracellular Traps in PAD4-Deficient Mice Contributes to Aggravated Acute Inflammation After Myocardial Infarction[J]. Front Immunol, 2019, 10: 2313. doi: 10.3389/fimmu.2019.02313
[7] Immler R, Simon SI, Sperandio M. Calcium signalling and related ion channels in neutrophil recruitment and function[J]. Eur J Clin Invest, 2018, 48(Suppl 2): e12964.
[8] Douda DN, Khan MA, Grasemann H, et al. SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx[J]. Proc Natl Acad Sci U S A, 2015, 112(9): 2817-2822. doi: 10.1073/pnas.1414055112
[9] Ravindran M, Khan MA, Palaniyar N. Neutrophil Extracellular Trap Formation: Physiology, Pathology, and Pharmacology[J]. Biomolecules, 2019, 9(8): 365. doi: 10.3390/biom9080365
[10] Thiam HR, Wong SL, Wagner DD, et al. Cellular Mechanisms of NETosis[J]. Annu Rev Cell Dev Biol, 2020, 36: 191-218. doi: 10.1146/annurev-cellbio-020520-111016
[11] Skendros P, Mitroulis I, Ritis K. Autophagy in Neutrophils: From Granulopoiesis to Neutrophil Extracellular Traps[J]. Front Cell Dev Biol, 2018, 6: 109. doi: 10.3389/fcell.2018.00109
[12] Helseth R, Shetelig C, Andersen GØ, et al. Neutrophil Extracellular Trap Components Associate with Infarct Size, Ventricular Function, and Clinical Outcome in STEMI[J]. Mediators Inflamm, 2019: 7816491.
[13] Du M, Yang W, Schmull S, et al. Inhibition of peptidyl arginine deiminase-4 protects against myocardial infarction induced cardiac dysfunction[J]. Int Immunopharmacol, 2020, 78: 106055. doi: 10.1016/j.intimp.2019.106055
[14] Vogel B, Shinagawa H, Hofmann U, et al. Acute DNase1 treatment improves left ventricular remodeling after myocardial infarction by disruption of free chromatin[J]. Basic Res Cardiol, 2015, 110(2): 15. doi: 10.1007/s00395-015-0472-y
[15] Chen C, Zhang H, Xie R, et al. Gut microbiota aggravate cardiac ischemia-reperfusion injury via regulating the formation of neutrophils extracellular traps[J]. Life Sci, 2022, 303: 120670. doi: 10.1016/j.lfs.2022.120670
[16] Abrams ST, Zhang N, Dart C, et al. Human CRP defends against the toxicity of circulating histones[J]. J Immunol, 2013, 191(5): 2495-2502. doi: 10.4049/jimmunol.1203181
[17] Friggeri A, Banerjee S, Xie N, et al. Extracellular histones inhibit efferocytosis[J]. Mol Med, 2012, 18(1): 825-833.
[18] Li YW, Chen SX, Yang Y, et al. Colchicine Inhibits NETs and Alleviates Cardiac Remodeling after Acute Myocardial Infarction[J]. Cardiovasc Drugs Ther, 2024, 38(1): 31-41. doi: 10.1007/s10557-022-07326-y
[19] Chrysanthopoulou A, Mitroulis I, Apostolidou E, et al. Neutrophil extracellular traps promote differentiation and function of fibroblasts[J]. J Pathol, 2014, 233(3): 294-307. doi: 10.1002/path.4359
[20] Hofbauer TM, Mangold A, Scherz T, et al. Neutrophil extracellular traps and fibrocytes in ST-segment elevation myocardial infarction[J]. Basic Res Cardiol, 2019, 114(5): 33. doi: 10.1007/s00395-019-0740-3
[21] Zhang Z, Ding S, Wang Z, et al. Prmt1 upregulated by Hdc deficiency aggravates acute myocardial infarction via NETosis[J]. Acta Pharm Sin B, 2022, 12(4): 1840-1855. doi: 10.1016/j.apsb.2021.10.016
[22] Mollenhauer M, Friedrichs K, Lange M, et al. Myeloperoxidase Mediates Postischemic Arrhythmogenic Ventricular Remodeling[J]. Circ Res, 2017, 121(1): 56-70. doi: 10.1161/CIRCRESAHA.117.310870
[23] Rudolph V, Andrié RP, Rudolph TK, et al. Myeloperoxidase acts as a profibrotic mediator of atrial fibrillation[J]. Nat Med, 2010, 16(4): 470-474. doi: 10.1038/nm.2124
[24] Krause KH, Welsh MJ. Voltage-dependent and Ca2(+)-activated ion channels in human neutrophils[J]. J Clin Invest, 1990, 85(2): 491-498. doi: 10.1172/JCI114464
[25] Fay AJ, Qian X, Jan YN, et al. SK channels mediate NADPH oxidase-independent reactive oxygen species production and apoptosis in granulocytes[J]. Proc Natl Acad Sci U S A, 2006, 103(46): 17548-17553. doi: 10.1073/pnas.0607914103
[26] Mazzoleni V, Zimmermann K, Smirnova A, et al. Staphylococcus aureus Panton-Valentine Leukocidin triggers an alternative NETosis process targeting mitochondria[J]. FASEB J, 2021, 35(2): e21167.
[27] Tackenberg H, Möller S, Filippi MD, et al. The Small GTPase Cdc42 Negatively Regulates the Formation of Neutrophil Extracellular Traps by Engaging Mitochondria[J]. Front Immunol, 2021, 12: 564720. doi: 10.3389/fimmu.2021.564720
[28] Hundahl LA, Sattler SM, Skibsbye L, et al. Pharmacological blockade of small conductance Ca2+-activated K+ channels by ICA reduces arrhythmic load in rats with acute myocardial infarction[J]. Pflugers Arch, 2017, 469(5-6): 739-750. doi: 10.1007/s00424-017-1962-6
[29] Takahashi M, Yokoshiki H, Mitsuyama H, et al. SK channel blockade prevents hypoxia-induced ventricular arrhythmias through inhibition of Ca2+/voltage uncoupling in hypertrophied hearts[J]. Am J Physiol Heart Circ Physiol, 2021, 320(4): H1456-H1469. doi: 10.1152/ajpheart.00777.2020
[30] Najder K, Musset B, Lindemann O, et al. The function of TRP channels in neutrophil granulocytes[J]. Pflugers Arch, 2018, 470(7): 1017-1033. doi: 10.1007/s00424-018-2146-8
[31] Massullo P, Sumoza-Toledo A, Bhagat H, et al. TRPM channels, calcium and redox sensors during innate immune responses[J]. Semin Cell Dev Biol, 2006, 17(6): 654-666. doi: 10.1016/j.semcdb.2006.11.006
[32] Qian X, Zhao H, Chen X, et al. Disruption of transient receptor potential melastatin 2 decreases elastase release and bacterial clearance in neutrophils[J]. Innate Immun, 2018, 24(2): 122-130. doi: 10.1177/1753425918759181
[33] Chauhan A, Sharma A, Tripathi JK, et al. Helminth derived factors inhibit neutrophil extracellular trap formation and inflammation in bacterial peritonitis[J]. Sci Rep, 2021, 11(1): 12718. doi: 10.1038/s41598-021-92001-9
[34] Tripathi JK, Sharma A, Sukumaran P, et al. Oxidant sensor cation channel TRPM2 regulates neutrophil extracellular trap formation and protects against pneumoseptic bacterial infection[J]. FASEB J, 2018, 32(12): fj201800605.
[35] Hiroi T, Wajima T, Negoro T, et al. Neutrophil TRPM2 channels are implicated in the exacerbation of myocardial ischaemia/reperfusion injury[J]. Cardiovasc Res, 2013, 97(2): 271-281. doi: 10.1093/cvr/cvs332
[36] Nilius B, Szallasi A. Transient receptor potential channels as drug targets: from the science of basic research to the art of medicine[J]. Pharmacol Rev, 2014, 66(3): 676-814. doi: 10.1124/pr.113.008268
[37] Parenti A, De Logu F, Geppetti P, et al. What is the evidence for the role of TRP channels in inflammatory and immune cells?[J]. Br J Pharmacol, 2016, 173(6): 953-969. doi: 10.1111/bph.13392
[38] Yin J, Michalick L, Tang C, et al. Role of Transient Receptor Potential Vanilloid 4 in Neutrophil Activation and Acute Lung Injury[J]. Am J Respir Cell Mol Biol, 2016, 54(3): 370-383. doi: 10.1165/rcmb.2014-0225OC
[39] 卢凯. TRPV4调节中性粒细胞活化介导心肌缺血再灌注损伤机制的初步研究[D]. 华中科技大学, 2023.
[40] 王斌斌, 吴琼峰, 廖杰, 等. TRPV4通道与缺血再灌注损伤的研究进展[J]. 临床心血管病杂志, 2018, 34(7): 636-639. doi: 10.13294/j.aps.2015.0065
[41] Wu QF, Qian C, Zhao N, et al. Activation of transient receptor potential vanilloid 4 involves in hypoxia/reoxygenation injury in cardiomyocytes[J]. Cell Death Dis, 2017, 8(5): e2828. doi: 10.1038/cddis.2017.227
[42] Lecut C, Frederix K, Johnson DM, et al. P2X1 ion channels promote neutrophil chemotaxis through Rho kinase activation[J]. J Immunol, 2009, 183(4): 2801-2809. doi: 10.4049/jimmunol.0804007
[43] Wang X, Chen D. Purinergic Regulation of Neutrophil Function[J]. Front Immunol, 2018, 9: 399. doi: 10.3389/fimmu.2018.00399
[44] Wang X, Qin W, Xu X, et al. Endotoxin-induced autocrine ATP signaling inhibits neutrophil chemotaxis through enhancing myosin light chain phosphorylation[J]. Proc Natl Acad Sci U S A, 2017, 114(17): 4483-4488. doi: 10.1073/pnas.1616752114
[45] Alarcón P, Manosalva C, Quiroga J, et al. Oleic and Linoleic Acids Induce the Release of Neutrophil Extracellular Traps via Pannexin 1-Dependent ATP Release and P2X1 Receptor Activation[J]. Front Vet Sci, 2020, 7: 260. doi: 10.3389/fvets.2020.00260
[46] Quiroga J, Alarcón P, Manosalva C, et al. Mitochondria-derived ATP participates in the formation of neutrophil extracellular traps induced by platelet-activating factor through purinergic signaling in cows[J]. Dev Comp Immunol, 2020, 113: 103768. doi: 10.1016/j.dci.2020.103768
[47] Lecut C, Faccinetto C, Delierneux C, et al. ATP-gated P2X1 ion channels protect against endotoxemia by dampening neutrophil activation[J]. J Thromb Haemost, 2012, 10(3): 453-465. doi: 10.1111/j.1538-7836.2011.04606.x
[48] Zhuang S, Xia S, Huang P, et al. Targeting P2RX1 alleviates renal ischemia/reperfusion injury by preserving mitochondrial dynamics[J]. Pharmacol Res, 2021, 170: 105712. doi: 10.1016/j.phrs.2021.105712
[49] Suh BC, Kim JS, Namgung U, et al. P2X7 nucleotide receptor mediation of membrane pore formation and superoxide generation in human promyelocytes and neutrophils[J]. J Immunol, 2001, 166(11): 6754-6763. doi: 10.4049/jimmunol.166.11.6754
[50] Kim SW, Davaanyam D, Seol SI, et al. Adenosine Triphosphate Accumulated Following Cerebral Ischemia Induces Neutrophil Extracellular Trap Formation[J]. Int J Mol Sci, 2020, 21(20): 7668. doi: 10.3390/ijms21207668
[51] Granado M, Amor S, Montoya JJ, et al. Altered expression of P2Y2 and P2X7 purinergic receptors in the isolated rat heart mediates ischemia-reperfusion injury[J]. Vascul Pharmacol, 2015, 73: 96-103. doi: 10.1016/j.vph.2015.06.003
[52] Painter RG, Valentine VG, Lanson NA Jr, et al. CFTR Expression in human neutrophils and the phagolysosomal chlorination defect in cystic fibrosis[J]. Biochemistry, 2006, 45(34): 10260-10269. doi: 10.1021/bi060490t
[53] Pohl K, Hayes E, Keenan J, et al. A neutrophil intrinsic impairment affecting Rab27a and degranulation in cystic fibrosis is corrected by CFTR potentiator therapy[J]. Blood, 2014, 124(7): 999-1009. doi: 10.1182/blood-2014-02-555268
[54] Gray RD, Hardisty G, Regan KH, et al. Delayed neutrophil apoptosis enhances NET formation in cystic fibrosis[J]. Thorax, 2018, 73(2): 134-144. doi: 10.1136/thoraxjnl-2017-210134
[55] Han H, Liu C, Li M, et al. Increased intracellular Cl-concentration mediates neutrophil extracellular traps formation in atherosclerotic cardiovascular diseases[J]. Acta Pharmacol Sin, 2022, 43(11): 2848-2861. doi: 10.1038/s41401-022-00911-9
[56] Clauzure M, Valdivieso AG, Massip Copiz MM, et al. Disruption of interleukin-1β autocrine signaling rescues complex I activity and improves ROS levels in immortalized epithelial cells with impaired cystic fibrosis transmembrane conductance regulator(CFTR)function[J]. PLoS One, 2014, 9(6): e99257. doi: 10.1371/journal.pone.0099257
[57] Clauzure M, Valdivieso ÁG, Dugour AV, et al. NLR family pyrin domain containing 3(NLRP3) and caspase 1(CASP1) modulation by intracellular Cl-concentration[J]. Immunology, 2021, 163(4): 493-511. doi: 10.1111/imm.13336
-




 下载:
下载: