Advancements in research regarding the involvement of neutrophils in myocardial ischemia-reperfusion injury
-
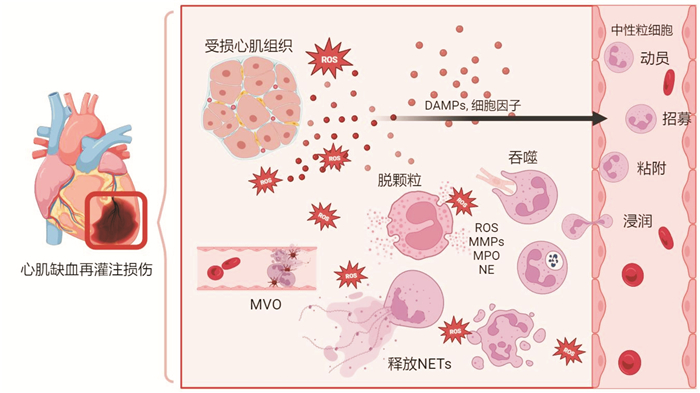
摘要: 中性粒细胞是心肌缺血再灌注后响应最迅速、数量最丰富的细胞,在启动炎症和组织修复中具有重要作用。本文重点概述了中性粒细胞的生理特点、生物学功能以及不同亚群之间的异质性,总结以中性粒细胞为中心介导的炎症级联反应在心肌缺血再灌注损伤进展中的重要作用,阐述多种靶向中性粒细胞调控的策略及其应用情况和转化前景。
-
关键词:
- 中性粒细胞 /
- 心肌缺血再灌注损伤 /
- 中性粒细胞胞外诱捕网 /
- 中性粒细胞亚群 /
- 炎症调控
Abstract: Neutrophils are the most responsive and abundant cells following myocardial ischemia-reperfusion, playing a crucial role in both inflammation and tissue repair. This paper provides a comprehensive summary of the physiological characteristics and biological functions of neutrophils, as well as the heterogeneity among different subpopulations. It also highlights the significant role of neutrophil-mediated inflammatory cascade in myocardial ischemia-reperfusion injury. Additionally, the paper reviews various regulatory strategies targeting neutrophils, discussing their application and potential for translation to clinical practice thus far. -

-
[1] Sun K, Li YY, Jin J. A double-edged sword of immuno-microenvironment in cardiac homeostasis and injury repair[J]. Signal Transduct Target Ther, 2021, 6: 79. doi: 10.1038/s41392-020-00455-6
[2] Heusch G. Myocardial ischaemia-reperfusion injury and cardioprotection in perspective[J]. Nat Rev Cardiol, 2020, 17: 773-789. doi: 10.1038/s41569-020-0403-y
[3] Carbone F, Bonaventura A, Montecucco F. Neutrophil-related oxidants drive heart and brain remodeling after ischemia/reperfusion injury[J]. Front Physiol, 2019, 10: 1587. doi: 10.3389/fpls.2019.01587
[4] Thiam HR, Wong SL, Wagner DD, et al. Cellular Mechanisms of NETosis[J]. Annu Rev Cell Dev Biol, 2020, 36: 191-218. doi: 10.1146/annurev-cellbio-020520-111016
[5] de Bont CM, Boelens WC, Pruijn GJM. NETosis, complement, and coagulation: a triangular relationship[J]. Cell Mol Immunol, 2019, 16: 19-27. doi: 10.1038/s41423-018-0024-0
[6] Cheng KH, Contreras GP, Yeh TY. Potential role of neutrophil extracellular traps in cardio-oncology[J]. Int J Mol Sci, 2022, 23(7): 3573. doi: 10.3390/ijms23073573
[7] Chen HM, Wu XH, Sun RC, et al. Dysregulation of CD177+ neutrophils on intraepithelial lymphocytes exacerbates gut inflammation via decreasing microbiota-derived DMF[J]. Gut Microbes, 2023, 15(1): 2172668. doi: 10.1080/19490976.2023.2172668
[8] Vafadarnejad E, Rizzo G, Krampert L, et al. Dynamics of cardiac neutrophil diversity in murine myocardial infarction[J]. Circ Res, 2020, 127(9): e232-e249.
[9] Hirano Y, Ode Y, Ochani M, et al. Targeting junctional adhesion molecule-C ameliorates sepsis-induced acute lung injury by decreasing CXCR4+ aged neutrophils[J]. J Leukoc Biol, 2018, 104(6): 1159-1171. doi: 10.1002/JLB.3A0218-050R
[10] Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation[J]. Nat Rev Immunol, 2013, 13: 159-175. doi: 10.1038/nri3399
[11] Qu JW, Jin JS, Zhang M, et al. Neutrophil diversity and plasticity: Implications for organ transplantation[J]. Cell Mol Immunol, 2023, 20(9): 993-1001. doi: 10.1038/s41423-023-01058-1
[12] Chapple ILC, Hirschfeld J, Kantarci A, et al. The role of the host-Neutrophil biology[J]. Periodontol 2000, 2023: Online ahead of print.
[13] Sreejit G, Abdel-Latif A, Athmanathan B, et al. Neutrophil-derived S100A8/A9 amplify granulopoiesis after myocardial infarction[J]. Circulation, 2020, 141(13): 1080-1094. doi: 10.1161/CIRCULATIONAHA.119.043833
[14] Mauler M, Herr N, Schoenichen C, et al. Platelet serotonin aggravates myocardial ischemia/reperfusion injury via neutrophil degranulation[J]. Circulation, 2019, 139(7): 918-931. doi: 10.1161/CIRCULATIONAHA.118.033942
[15] Wang LT, Wang HH, Chiang HC, et al. Human placental MSC-secreted IL-1β enhances neutrophil bactericidal functions during hypervirulent Klebsiella infection[J]. Cell Rep, 2020, 32(13): 108188. doi: 10.1016/j.celrep.2020.108188
[16] Hofbauer TM, Ondracek AS, Mangold A, et al. Neutrophil extracellular traps induce MCP-1 at the culprit site in ST-segment elevation myocardial infarction[J]. Front Cell Dev Biol, 2020, 8: 564169. doi: 10.3389/fcell.2020.564169
[17] Sharma DJ Sr, Nath HJ, Batta A, et al. Neutrophil-to-lymphocyte ratio(NLR)useful as a cost-effective preliminary prognostic marker in ST-elevation myocardial infarction(STEMI): an observational study from a tertiary care hospital in northeast India[J]. Cureus, 2023, 15(3): e36885.
[18] Yndestad A, Landrø L, Ueland T, et al. Increased systemic and myocardial expression of neutrophil gelatinase-associated lipocalin in clinical and experimental heart failure[J]. Eur Heart J, 2009, 30(10): 1229-1236. doi: 10.1093/eurheartj/ehp088
[19] Chaar D, Dumont B, Vulesevic B, et al. Neutrophils pro-inflammatory and anti-inflammatory cytokine release in patients with heart failure and reduced ejection fraction[J]. ESC Heart Fail, 2021, 8(5): 3855-3864. doi: 10.1002/ehf2.13539
[20] Liu SF, Ngo DTM, Chong CR, et al. Suppression of neutrophil superoxide generation by BNP is attenuated in acute heart failure: a case for'BNP resistance'[J]. Eur J Heart Fail, 2015, 17(5): 475-483. doi: 10.1002/ejhf.242
[21] Dehghani T, Thai PN, Sodhi H, et al. Selectin-targeting glycosaminoglycan-peptide conjugate limits neutrophil-mediated cardiac reperfusion injury[J]. Cardiovasc Res, 2022, 118(1): 267-281. doi: 10.1093/cvr/cvaa312
[22] Zhang XJ, Liu S, Weng XY, et al. Brg1 deficiency in vascular endothelial cells blocks neutrophil recruitment and ameliorates cardiac ischemia-reperfusion injury in mice[J]. Int J Cardiol, 2018, 269: 250-258. doi: 10.1016/j.ijcard.2018.07.105
[23] Zhang RYK, Cochran BJ, Thomas SR, et al. Impact of reperfusion on temporal immune cell dynamics after myocardial infarction[J]. J Am Heart Assoc, 2023, 12(4): e027600. doi: 10.1161/JAHA.122.027600
[24] Li JW, Conrad C, Mills TW, et al. PMN-derived netrin-1 attenuates cardiac ischemia-reperfusion injury via myeloid ADORA2B signaling[J]. J Exp Med, 2021, 218(6): e20210008. doi: 10.1084/jem.20210008
[25] Horckmans M, Ring L, Duchene J, et al. Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype[J]. Eur Heart J, 2017, 38(3): 187-197.
[26] Ma YG, Yabluchanskiy A, Iyer RP, et al. Temporal neutrophil polarization following myocardial infarction[J]. Cardiovasc Res, 2016, 110(1): 51-61. doi: 10.1093/cvr/cvw024
[27] Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease[J]. N Engl J Med, 2017, 377(12): 1119-1131. doi: 10.1056/NEJMoa1707914
[28] Marinković G, Grauen Larsen H, Yndigegn T, et al. Inhibition of pro-inflammatory myeloid cell responses by short-term S100A9 blockade improves cardiac function after myocardial infarction[J]. Eur Heart J, 2019, 40(32): 2713-2723. doi: 10.1093/eurheartj/ehz461
[29] Marinković G, Koenis DS, de Camp L, et al. S100A9 links inflammation and repair in myocardial infarction[J]. Circ Res, 2020, 127(5): 664-676. doi: 10.1161/CIRCRESAHA.120.315865
[30] Braunersreuther V, Pellieux C, Pelli G, et al. Chemokine CCL5/RANTES inhibition reduces myocardial reperfusion injury in atherosclerotic mice[J]. J Mol Cell Cardiol, 2010, 48(4): 789-798. doi: 10.1016/j.yjmcc.2009.07.029
[31] Lemarié J, Boufenzer A, Popovic B, et al. Pharmacological inhibition of the triggering receptor expressed on myeloid cells-1 limits reperfusion injury in a porcine model of myocardial infarction[J]. ESC Heart Fail, 2015, 2(2): 90-99. doi: 10.1002/ehf2.12029
[32] George MJ, Jasmin NH, Cummings VT, et al. Selective interleukin-6 trans-signaling blockade is more effective than panantagonism in reperfused myocardial infarction[J]. JACC Basic Transl Sci, 2021, 6(5): 431-443. doi: 10.1016/j.jacbts.2021.01.013
[33] Rose-John S, Jenkins BJ, Garbers C, et al. Targeting IL-6 trans-signalling: past, present and future prospects[J]. Nat Rev Immunol, 2023, 23(10): 666-681. doi: 10.1038/s41577-023-00856-y
[34] Dillemans L, De Somer L, Neerinckx B, et al. A review of the pleiotropic actions of the IFN-inducible CXC chemokine receptor 3 ligands in the synovial microenvironment[J]. Cell Mol Life Sci, 2023, 80(3): 78. doi: 10.1007/s00018-023-04715-w
[35] García-Prieto J, Villena-Gutiérrez R, Gómez M, et al. Neutrophil stunning by metoprolol reduces infarct size[J]. Nat Commun, 2017, 8: 14780. doi: 10.1038/ncomms14780
[36] Vajen T, Koenen RR, Werner I, et al. Blocking CCL5-CXCL4 heteromerization preserves heart function after myocardial infarction by attenuating leukocyte recruitment and NETosis[J]. Sci Rep, 2018, 8: 10647. doi: 10.1038/s41598-018-29026-0
[37] Du MJ, Yang WG, Schmull S, et al. Inhibition of peptidyl arginine deiminase-4 protects against myocardial infarction induced cardiac dysfunction[J]. Int Immunopharmacol, 2020, 78: 106055. doi: 10.1016/j.intimp.2019.106055
[38] Bonilha CS, Veras FP, de Queiroz Cunha F. NET-targeted therapy: effects, limitations, and potential strategies to enhance treatment efficacy[J]. Trends Pharmacol Sci, 2023, 44(9): 622-634. doi: 10.1016/j.tips.2023.06.007
[39] Zhang Y, Jian W, He L, et al. Externalized histone H4: a novel target that orchestrates chronic inflammation by inducing lytic cell death[J]. Acta Biochim Biophys Sin(Shanghai), 2020, 52(3): 336-338.
[40] Gupta A, Singh K, Fatima S, et al. Neutrophil extracellular traps promote NLRP3 inflammasome activation and glomerular endothelial dysfunction in diabetic kidney disease[J]. Nutrients, 2022, 14(14): 2965. doi: 10.3390/nu14142965
[41] Gao RF, Shi HR, Chang SC, et al. The selective NLRP3-inflammasome inhibitor MCC950 reduces myocardial fibrosis and improves cardiac remodeling in a mouse model of myocardial infarction[J]. Int Immunopharmacol, 2019, 74: 105575. doi: 10.1016/j.intimp.2019.04.022
[42] Zhu SS, Zhang JQ, Xue NR, et al. Highly specific neutrophil-mediated delivery of albumin nanoparticles to ectopic lesion for endometriosis therapy[J]. J Nanobiotechnol, 2023, 21(1): 81. doi: 10.1186/s12951-023-01831-4
[43] Irwandi RA, Chiesa ST, Hajishengallis G, et al. The roles of neutrophils linking periodontitis and atherosclerotic cardiovascular diseases[J]. Front Immunol, 2022, 13: 915081. doi: 10.3389/fimmu.2022.915081
[44] Nidorf S, Fiolet A, Mosterd A, et al. Colchicine in patients with chronic coronary disease[J]. N Engl J Med, 2020, 383: 1838-1847. doi: 10.1056/NEJMoa2021372
[45] Imazio M, Nidorf M. Colchicine and the heart[J]. Eur Heart J, 2021, 42(28): 2745-2760. doi: 10.1093/eurheartj/ehab221
[46] Sun X, Duan JF, Gong CY, et al. Colchicine ameliorates dilated cardiomyopathy via SIRT2-mediated suppression of NLRP3 inflammasome activation[J]. J Am Heart Assoc, 2022, 11(13): e025266. doi: 10.1161/JAHA.122.025266
-




 下载:
下载: