Early initiation of evolocumab in patients with non-ST-segment elevation acute coronary syndrome undergoing percutaneous coronary intervention
-
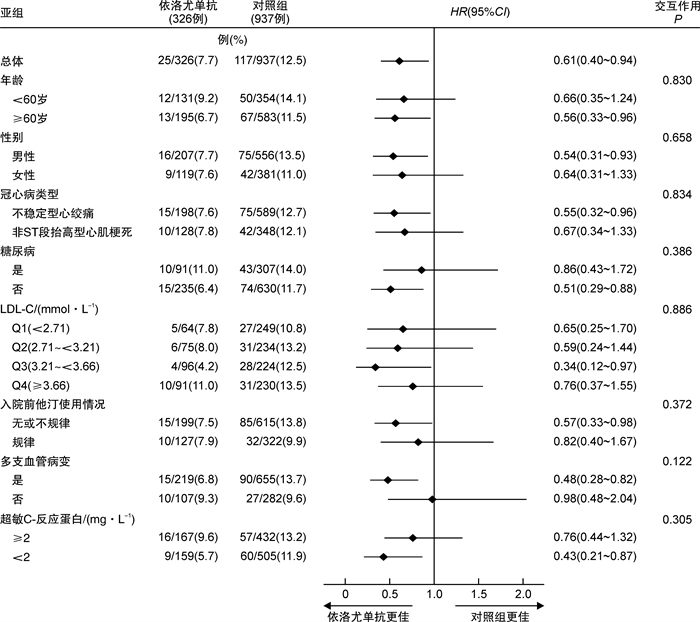
摘要: 目的 探讨非ST段抬高型急性冠状动脉综合征(NSTE-ACS)患者经皮冠状动脉介入治疗(PCI)后早期应用依洛尤单抗的有效性和安全性。方法 回顾性连续纳入2019年3月1日—2020年5月31日于郑州大学第一附属医院心血管内科确诊的NSTE-ACS、接受PCI治疗同时伴随高低密度脂蛋白胆固醇(LDL-C)水平的患者1263例(既往规律服用4周以上他汀类药物者LDL-C≥1.8 mmol/L;既往未服用或未规律服用他汀类药物者LDL-C≥3.0 mmol/L)。根据药物使用分为依洛尤单抗组(326例)和对照组(937例)。依洛尤单抗组患者于PCI术后院内早期应用依洛尤单抗(每2周皮下注射140 mg)。2组患者均规律使用他汀类药物治疗。收集2组患者基线以及出院后1、6、12、18个月的LDL-C水平。主要终点为随访18个月的心血管不良事件(包括心血管死亡、心肌梗死、缺血性卒中、因不稳定型心绞痛住院和冠状动脉血运重建在内的复合终点)。同时在8个预先设定的亚组中评估依洛尤单抗对主要终点的影响。结果 与对照组相比,依洛尤单抗联合他汀类药物在第18个月随访时可将LDL-C水平较基线降低42.54%。经多因素Cox回归校正后,在他汀治疗的基础上早期应用依洛尤单抗可显著降低主要终点事件发生率(7.7% vs 12.5%;HR=0.61;95%CI:0.40~0.94;P=0.025)。亚组分析表明,依洛尤单抗在各个亚组中可显著降低主要终点事件的发生率,该结果与总体分析一致。结论 在NSTE-ACS行PCI治疗的患者中,在他汀治疗的基础上早期联用依洛尤单抗可显著降低LDL-C水平及心血管事件的发生风险,其疗效持续且安全性良好。
-
关键词:
- 非ST段抬高型急性冠状动脉综合征 /
- 依洛尤单抗 /
- 低密度脂蛋白胆固醇 /
- 经皮冠状动脉介入治疗
Abstract: Objective To evaluate the efficacy and safety of the early initiation of evolocumab in Chinese patients with non-ST-segment elevation acute coronary syndrome(NSTE-ACS) undergoing percutaneous coronary intervention(PCI).Methods We performed a retrospective study involving 1263 consecutive patients with NSTE-ACS undergoing PCI, and who either had an LDL-C level of at least 1.8 mmol/L after receiving statins for more than 4 weeks or an LDL-C level of at least 3.0 mmol/L without regular statin treatment. They were divided into the evolocumab group(n=326) and the control group(n=937) according to whether evolocumab was used or not. Evolocumab was initiated in the hospital and administered for 18 months after discharge. All enrolled patients received regular statin treatment. LDL-C levels were measured at baseline and 1, 6, 12 and 18 months after discharge. The primary endpoint at 18 months was a composite of ischemic stroke, cardiovascular death, myocardial infarction, hospitalization for unstable angina or coronary revascularization. The effect of evolocumab on the primary endpoint was evaluated in 8 prespecified subgroups.Results The addition of evolocumab to statin treatment lowered LDL-C levels by 42.54% from baseline compared with statins alone. Early initiation of evolocumab on a background of statin treatment was associated with a significant reduction in the primary endpoint(7.7% vs. 12.5%;HR, 0.61; 95%CI, 0.40~0.94;P=0.025). In subgroup analyses, the significant reduction in the primary endpoint with evolocumab treatment was consistent across all prespecified subgroups.Conclusion Among Chinese patients with NSTE-ACS undergoing PCI, the early initiation of evolocumab on a background of statin therapy significantly lowered LDL-C levels and the risk of adverse cardiovascular events, with sustained efficacy and satisfactory safety. -

-
表 1 基线资料
Table 1. Characteristics of the patients at baseline
例(%), X±S 项目 依洛尤单抗组(326例) 对照组(937例) χ2/t P 年龄/岁 62.3±10.8 62.4±10.0 0.026 0.979 男性 207(63.5) 556(59.3) 1.749 0.186 体重/kg 72.7±11.8 73.9±12.1 1.539 0.124 冠心病类型 0.465 0.495 不稳定型心绞痛 198(60.7) 589(62.9) NSTEMI 128(39.3) 348(37.1) 术前心搏骤停 8(2.5) 18(1.9) 0.341 0.559 高血压 214(65.6) 614(65.5) 0.001 0.970 吸烟 114(35.0) 300(32.0) 0.957 0.328 糖尿病 91(27.9) 307(32.8) 2.636 0.104 胰岛素依赖 33(10.1) 102(10.9) 0.148 0.701 卒中史 16(4.9) 75(8.0) 3.468 0.063 心肌梗死史 42(12.9) 140(14.9) 0.830 0.362 PCI史 63(19.3) 194(20.7) 0.284 0.594 CABG史 11(3.4) 30(3.2) 0.023 0.880 冠心病家族史 90(27.6) 212(22.6) 3.299 0.069 周围血管病 17(5.2) 32(3.4) 2.100 0.147 COPD 20(6.1) 67(7.2) 0.389 0.533 入院前他汀使用情况 2.226 0.136 规律 127(39.0) 322(34.4) 无或不规律 199(61.0) 615(65.6) 肾小球滤过率/(mL·min-1) 84.0±19.1 85.4±23.0 1.083 0.279 CABG:冠脉旁路移植术;COPD:慢性阻塞性肺疾病。 表 2 手术特征
Table 2. Procedural characteristics
例(%), X±S 手术特征 依洛尤单抗组(326例) 对照组(937例) χ2/t P 穿刺入路 3.918 0.048 桡动脉 286(87.7) 857(91.5) 股动脉 40(12.3) 80(8.5) IABP 12(3.7) 27(2.9) 0.517 0.472 血运重建策略 2.947 0.086 支架植入 311(95.4) 912(97.3) 单纯球囊扩张 15(4.6) 25(2.7) 干预血管部位 LM 20(6.1) 88(9.4) 3.280 0.070 LAD 174(53.4) 547(58.4) 2.472 0.116 LCX 92(28.2) 270(28.8) 0.042 0.838 RCA 129(39.6) 329(35.1) 2.080 0.149 ≥2支血管接受处理 82(25.2) 258(27.5) 0.697 0.404 术前TIMI血流0或1级 113(34.7) 305(32.6) 0.487 0.485 病变血管数目 0.846 0.655 单支 107(32.8) 282(30.1) 双支 78(23.9) 232(24.8) 三支 141(43.3) 423(45.1) 支架数目/个 1.85±0.87 1.88±0.96 0.475 0.635 支架长度/mm 45.42±27.60 46.21±29.82 0.416 0.677 完全手术成功 315(96.6) 917(97.9) 1.553 0.213 术中抗凝药 2.896 0.089 比伐芦定 142(43.6) 358(38.2) 肝素 184(56.4) 579(61.8) IABP:主动脉内球囊反搏;LM:左主干;LAD:左前降支;LCX:左回旋支;RCA:右冠脉;TIMI:心肌梗死溶栓试验。 表 3 2组患者治疗前后LDL-C变化比较
Table 3. Comparison of LDL-C changes in the two groups before and after treatment
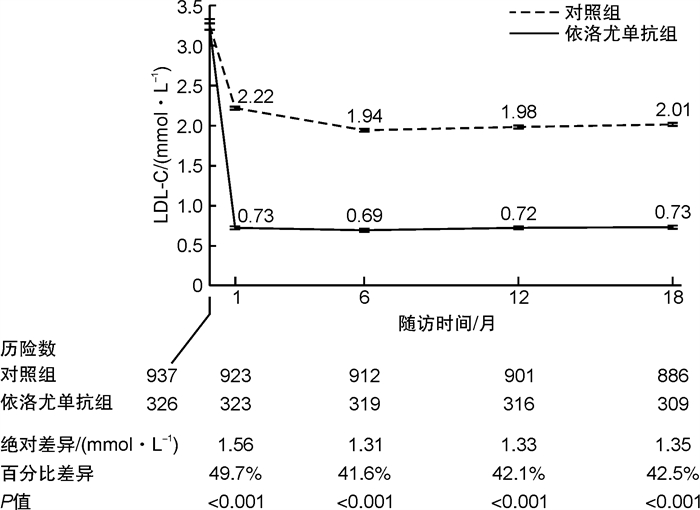
例(%), X±S LDL-C 依洛尤单抗组(326例) 对照组(937例) 平均差(95%CI) χ2/t P 基线/(mmol·L-1) 3.31±0.71 3.24±0.76 -0.07(-0.17~0.02) 1.51 0.132 治疗第1个月/(mmol·L-1) 0.73±0.41 2.22±0.49 1.49(1.44~1.55) 53.62 < 0.001 基线至第1个月的绝对变化/(mmol·L-1) -2.58±0.77 -1.02±0.87 1.56(1.46~1.67) 30.17 < 0.001 基线至第1个月的百分比变化/% -77.25±13.47 -27.54±24.02 49.71(47.57~51.85) 45.64 < 0.001 治疗第18个月/(mmol·L-1) 0.73±0.44 2.01±0.49 1.28(1.22~1.34) 42.36 < 0.001 基线至第18个月的绝对变化/(mmol·L-1) -2.58±0.80 -1.23±0.87 1.35(1.24~1.45) 24.90 < 0.001 基线至第18个月的百分比变化/% -77.00±14.64 -34.46±22.67 42.54(40.33~44.76) 37.69 < 0.001 治疗第18个月LDL-C < 1.8 mmol/L 298(96.4) 342(38.6) - 308.13 < 0.001 治疗第18个月LDL-C < 1.4 mmol/L 287(92.9) 94(10.6) - 714.01 < 0.001 表 4 随访第18个月的临床结局
Table 4. Clinical outcomes up to 18 months
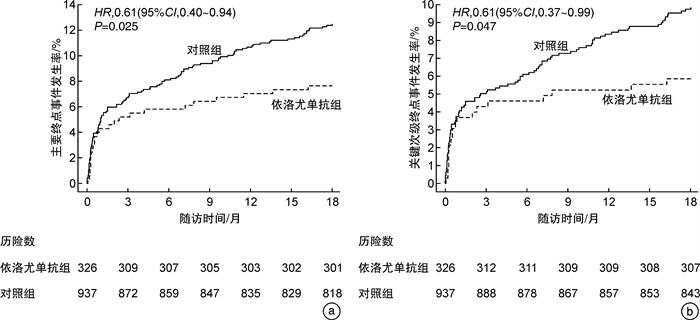
例(%) 临床结局 依洛尤单抗组(326例) 对照组(937例) HR(95%CI) P 主要终点 25(7.7) 117(12.5) 0.61(0.40~0.94) 0.025 关键次级终点 19(5.8) 92(9.8) 0.61(0.37~0.99) 0.047 心血管死亡 3(0.9) 20(2.1) 0.43(0.13~1.44) 0.170 全因死亡 3(0.9) 24(2.6) 0.37(0.11~1.23) 0.106 缺血性卒中 6(1.8) 15(1.6) 1.14(0.44~2.95) 0.782 心肌梗死 12(3.7) 64(6.9) 0.54(0.29~1.01) 0.053 因不稳定型心绞痛住院 2(0.6) 11(1.2) 0.52(0.12~2.36) 0.399 冠脉血运重建 21(6.5) 70(7.5) 0.85(0.52~1.39) 0.519 注:表中百分比为临床结局的累积事件发生率(Kaplan-Meier估计值)。 表 5 不良事件与实验室检查结果
Table 5. Adverse events and laboratory test results
例(%) 安全终点 依洛尤单抗组(326例) 对照组(937例) χ2 P 不良事件 肌肉相关症状 12(3.7) 27(2.9) 0.517 0.472 新发糖尿病 7(2.1) 24(2.6) 0.173 0.677 神经认知功能障碍 2(0.6) 8(0.9) 0.178 0.673 新发白内障 2(0.6) 7(0.7) 0.061 0.805 实验室异常 转氨酶水平>3倍正常值上限 5/317(1.6) 15/908(1.7) 0.008 0.928 肌酸激酶水平>5倍正常值上限 1/316(0.3) 5/905(0.6) 0.267 0.605 -
[1] 石磊, 陈万, 李庆宽, 等. 非ST段抬高型急性心肌梗死高危患者早期及晚期介入治疗对长期预后的影响[J]. 临床急诊杂志, 2021, 22(4): 252-255, 260. https://www.cnki.com.cn/Article/CJFDTOTAL-ZZLC202104006.htm
[2] Jernberg T, Hasvold P, Henriksson M, et al. Cardiovascular risk in post-myocardial infarction patients: nationwide real world data demonstrate the importance of a long-term perspective[J]. Eur Heart J, 2015, 36(19): 1163-1170. doi: 10.1093/eurheartj/ehu505
[3] Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology(ESC)[J]. Eur Heart J, 2016, 37(3): 267-315. doi: 10.1093/eurheartj/ehv320
[4] Borén J, Chapman MJ, Krauss RM, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel[J]. Eur Heart J, 2020, 41(24): 2313-2330. doi: 10.1093/eurheartj/ehz962
[5] Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk[J]. Eur Heart J, 2020, 41(1): 111-188.
[6] Jiang J, Zhou YJ, Li JJ, et al. Uncontrolled hyperlipidemia in Chinese patients who experienced acute coronary syndrome: an observational study[J]. Ther Clin Risk Manag, 2018, 14: 2255-2264. doi: 10.2147/TCRM.S178318
[7] Sabatine MS, Giugliano RP, Keech A, et al. Rationale and design of the Further cardiovascular OUtcomes Research with PCSK9 Inhibition in subjects with Elevated Risk trial[J]. Am Heart J, 2016, 173: 94-101. doi: 10.1016/j.ahj.2015.11.015
[8] Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease[J]. N Engl J Med, 2017, 376(18): 1713-1722. doi: 10.1056/NEJMoa1615664
[9] Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events[J]. N Engl J Med, 2015, 372(16): 1500-1509. doi: 10.1056/NEJMoa1500858
[10] Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines[J]. Circulation, 2019, 139(25): e1082-e1143.
[11] 彭道泉, 杨阳. 血脂管理与ASCVD的回顾与展望[J]. 临床心血管病杂志, 2020, 36(9): 783-786. https://www.cnki.com.cn/Article/CJFDTOTAL-LCXB202009001.htm
[12] Cholesterol Treatment Trialists'(CTT)Collaboration, Baigent C, Blackwell L, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170, 000 participants in 26 randomised trials[J]. Lancet, 2010, 376(9753): 1670-1681. doi: 10.1016/S0140-6736(10)61350-5
[13] Bruckert E, Hayem G, Dejager S, et al. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients--the PRIMO study[J]. Cardiovasc Drugs Ther, 2005, 19(6): 403-414. doi: 10.1007/s10557-005-5686-z
[14] Cohen JD, Brinton EA, Ito MK, et al. Understanding Statin Use in America and Gaps in Patient Education(USAGE): an internet-based survey of 10, 138 current and former statin users[J]. J Clin Lipidol, 2012, 6(3): 208-215. doi: 10.1016/j.jacl.2012.03.003
[15] Preiss D, Seshasai SR, Welsh P, et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis[J]. JAMA, 2011, 305(24): 2556-2564. doi: 10.1001/jama.2011.860
[16] HPS2-THRIVE Collaborative Group. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment[J]. Eur Heart J, 2013, 34(17): 1279-1291.
[17] Gitt AK, Lautsch D, Ferrieres J, et al. Low-density lipoprotein cholesterol in a global cohort of 57, 885 statin-treated patients[J]. Atherosclerosis, 2016, 255: 200-209.
[18] Zhao S, Wang Y, Mu Y, et al. Prevalence of dyslipidaemia in patients treated with lipid-lowering agents in China: results of the DYSlipidemia International Study(DYSIS)[J]. Atherosclerosis, 2014, 235(2): 463-469.
[19] Chiang CE, Ferrières J, Gotcheva NN, et al. Suboptimal Control of Lipid Levels: Results from 29 Countries Participating in the Centralized Pan-Regional Surveys on the Undertreatment of Hypercholesterolaemia(CEPHEUS)[J]. J Atheroscler Thromb, 2016, 23(5): 567-587.
[20] Fox KA, Dabbous OH, Goldberg RJ, et al. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: prospective multinational observational study(GRACE)[J]. BMJ, 2006, 333(7578): 1091.
[21] 隗沫, 顾伟, 李昭, 等. 心电图aVR导联对急性非ST段抬高型心肌梗死的病变血管的预测价值及预后评估[J]. 临床急诊杂志, 2021, 22(7): 487-490. https://www.cnki.com.cn/Article/CJFDTOTAL-ZZLC202107010.htm
[22] Nicholls SJ, Nissen SE, Prati F, et al. Assessing the impact of PCSK9 inhibition on coronary plaque phenotype with optical coherence tomography: rationale and design of the randomized, placebo-controlled HUYGENS study[J]. Cardiovasc Diagn Ther, 2021, 11(1): 120-129.
[23] Koskinas KC, Windecker S, Pedrazzini G, et al. Evolocumab for Early Reduction of LDL Cholesterol Levels in Patients With Acute Coronary Syndromes(EVOPACS)[J]. J Am Coll Cardiol, 2019, 74(20): 2452-2462.
[24] Guedeney P, Giustino G, Sorrentino S, et al. Efficacy and safety of alirocumab and evolocumab: a systematic review and meta-analysis of randomized controlled trials[J]. Eur Heart J, 2019.
-




 下载:
下载: