Efficacy analysis at 3-year follow-up of drug-coated balloons in the treatment of coronary de novo diffuse lesions
-
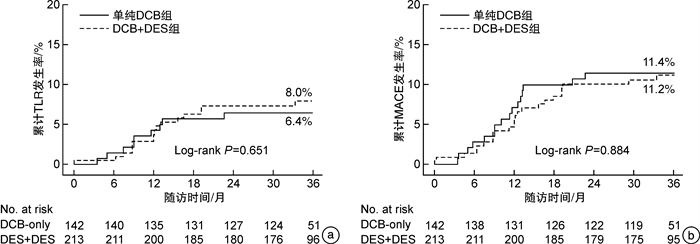
摘要: 目的 探讨药物涂层球囊(DCB)治疗冠状动脉(冠脉)原位弥漫性病变的有效性和安全性。方法 本研究是一项单中心、前瞻性的观察性研究,连续纳入2015年1月—2019年12月郑州大学第一附属医院心血管内科接受DCB治疗的冠脉原位弥漫性病变(病变长度>25 mm)患者355例,将其分为单纯DCB组(142例)和DCB+DES组(213例)。主要终点是3年临床随访中的靶病变再次血运重建(TLR),次要终点是主要心血管不良事件(MACE),包括靶血管再次血运重建、靶病变内血栓、非致死性心肌梗死和死亡。结果 与DCB+DES组比较,单纯DCB组病变长度更短[(36.55±13.27) mm vs (48.12±16.76) mm,P< 0.001]、参考血管直径更小[(2.33±0.44) mm vs (2.57±0.44) mm,P< 0.001]。单纯DCB组单个病变使用DCB个数更多[(1.66±0.68)个vs(1.10±0.30)个,P< 0.001],DCB长度更长[(44.43±17.70) mm vs (26.31±11.01) mm,P< 0.001],DCB直径更小[(2.64±0.37) mm vs (2.72±0.39) mm,P=0.037]。单纯DCB组中有10处(6.99%)病变行补救性支架。临床随访显示单纯DCB组在主要终点3年TLR累计发生率(6.34%)上较DCB+DES组(7.51%)稍低,差异无统计学意义(log-rankP=0.651),在次要终点MACE累计发生率(11.27% vs 10.80%,log-rankP=0.884)差异无统计学意义。结论 3年临床随访显示单纯DCB和DCB联合DES策略治疗冠脉原位弥漫性病变是有效和安全的。
-
关键词:
- 冠心病 /
- 弥漫性病变 /
- 经皮冠状动脉介入治疗 /
- 药物涂层球囊 /
- 药物洗脱支架
Abstract: Objective To investigate the efficacy and safety of drug-coated balloon(DCB) in the treatment ofde novocoronary diffuse lesions.Methods This study is a single-center, prospective, observational study. A total of 355 patients with coronary diffuse lesions(lesion length>25 mm) who received DCB in the Department of Cardiovascular Medicine, The First Affiliated Hospital of Zhengzhou University from January 2015 to December 2019 were consecutively enrolled, divided into DCB-only group(142 cases) and DCB+DES group(213 cases). The primary endpoint was target lesion revascularization(TLR) at 3-year clinical follow-up, and the secondary endpoint was major adverse cardiovascular events(MACE), including target vessel revascularization, target lesion thrombosis, non-fatal myocardial infarction, and death.Results Compared with the DCB+DES group, the DCB-only group showed shorter lesion length [(36.55±13.27) mm vs(48.12±16.76) mm,P< 0.001], and smaller reference vessel diameter [(2.33±0.44) mm vs(2.57±0.44) mm,P< 0.001]. In the DCB-only group, the mean number of DCBs per lesion was more(1.66±0.68 vs 1.10±0.30,P< 0.001), the length of DCB was longer[(44.43±17.70) mm vs(26.31±11.01) mm,P< 0.001], and the diameter of DCB was smaller[(2.64±0.37) mm vs(2.72±0.39) mm,P=0.037]than the DCB+DES group. In the DCB-only group, 10 lesions(6.99%) received bailout stents. The 3-year clinical follow-up showed that the DCB-only group had a numerically lower TLR incidence(6.34%) than the DCB DES group(7.51%) but did not reach statistical significance(log-rankP=0.651), and the incidence of MACE(11.27% vs 10.80%, log-rankP=0.884) was not statistically different.Conclusion 3-year clinical follow-up shows that DCB alone and DCB combined with DES strategies are effective and safe in treatingde novocoronary diffuse lesions. -

-
表 1 一般临床资料比较
Table 1. General clinical data
例(%), X±S 项目 总体(355例) 单纯DCB组(142例) DCB+DES组(213例) P 男性 246(69.30) 97(68.31) 149(69.95) 0.814 年龄/岁 58.78±11.12 58.47±10.83 58.98±11.33 0.673 高血压 209(58.87) 82(57.75) 127(59.62) 0.742 糖尿病 113(31.83) 46(32.39) 67(31.46) 0.908 高脂血症 151(42.54) 70(49.30) 81(38.03) 0.038 多支病变 240(67.61) 99(69.72) 141(66.20) 0.563 吸烟史 151(42.54) 58(40.85) 93(43.66) 0.661 家族史 94(26.48) 43(30.28) 51(23.94) 0.220 卒中史 54(15.21) 25(17.61) 29(13.62) 0.366 既往MI 77(21.69) 35(24.65) 42(19.72) 0.294 既往PCI 49(13.80) 28(19.72) 21(9.86) 0.012 LVEF/% 58.28±8.03 58.40±8.29 58.21±7.86 0.823 阿司匹林 355(100) 142(100) 213(100) >0.999 氯吡格雷 126(35.49) 52(36.62) 74(34.74) 0.735 替格瑞洛 229(64.51) 90(63.38) 139(65.26) 0.735 GP Ⅱb/Ⅲa受体拮抗剂 235(66.20) 93(65.49) 142(66.67) 0.820 LVEF:左室射血分数。 表 2 病变特征和手术过程
Table 2. Patient interventional data
例(%) 项目 总体(360处) 单纯DCB组(143处) DCB+DES组(217处) P 动脉入路 0.059 桡动脉 311(86.39) 130(90.91) 181(83.41) 股动脉 49(13.61) 13(9.09) 36(16.59) 靶病变血管 < 0.001 LAD 175(48.61) 51(35.66) 124(57.14) LCX 81(22.50) 44(30.77) 37(17.05) RCA 104(28.89) 48(33.57) 56(25.81) CTO病变 136(37.78) 46(32.17) 90(41.47) 0.077 钙化 96(26.67) 29(20.28) 67(30.88) 0.029 旋磨 21(5.83) 5(3.50) 16(7.37) 0.168 IVUS 48(13.33) 13(9.09) 35(16.13) 0.059 预扩张 半顺应性球囊 341(94.72) 134(93.71) 207(95.39) 0.482 切割球囊 88(24.44) 28(19.58) 60(27.65) 0.103 棘突球囊 110(30.56) 54(37.76) 56(25.81) 0.019 双导丝球囊 5(1.39) 4(2.80) 1(0.46) 0.083 CTO:慢性晚期闭塞;LCX:左回旋支;RCA:右冠状动脉。 表 3 QCA结果
Table 3. QCA results
X±S 项目 总体(360处) 单纯DCB组(143处) DCB+DES组(217处) P 病变平均DCB个数/个 1.32±0.56 1.66±0.68 1.10±0.30 < 0.001 DCB长度/mm 33.51±16.60 44.43±17.70 26.31±11.01 < 0.001 DCB直径/mm 2.69±0.39 2.64±0.37 2.72±0.39 0.037 DCB扩张压力/atm 7.86±1.00 7.87±1.03 7.85±0.98 0.842 DCB扩张时间/s 59.28±3.13 59.48±2.57 59.15±3.45 0.331 病变平均DES个数/个 - - 1.42±0.58 - DES长度/mm - - 39.84±17.14 - DES直径/mm - - 2.84±0.31 - DCB+DES长度/mm 57.52±21.97 44.43±17.70 66.15±20.19 < 0.001 病变长度/mm 43.52±16.46 36.55±13.27 48.12±16.76 < 0.001 RVD/mm 2.47±0.45 2.33±0.44 2.57±0.44 < 0.001 MLD/mm 0.48±0.48 0.49±0.48 0.47±0.49 0.638 DS/% 80.78±19.09 79.30±19.62 81.75±18.72 0.234 DCB或DES术后即刻 RVD/mm 2.47±0.45 2.33±0.44 2.57±0.44 < 0.001 MLD/mm 1.79±0.46 1.62±0.39 1.90±0.47 < 0.001 DS/% 28.13±11.00 30.35±9.76 26.67±11.54 0.001 急性管腔获得/mm 1.31±0.61 1.13±0.55 1.43±0.62 < 0.001 补救性支架/例(%) 10(2.78) 10(6.99) - - 表 4 患者随访结果
Table 4. Clinical follow-up
例(%) 项目 总体(355例) 单纯DCB组(142例) DCB+DES组(213例) Log-rank P 围手术期 血栓 0(0) 0(0) 0(0) >0.999 MI 0(0) 0(0) 0(0) >0.999 死亡 1(0.28) 0(0) 1(0.47) >0.999 TLR 25(7.04) 9(6.34) 16(7.51) 0.651 TLR位置 DCB段 14(3.94) 9(6.34) 5(2.35) - DES段 7(1.97) - 7(3.29) - DCB和DES重叠段 4(1.13) - 4(1.88) - TVR 31(8.73) 14(9.86) 17(7.98) 0.544 全因死亡 9(2.53) 2(1.41) 7(3.29) 0.255 心源性死亡 6(1.69) 1(0.70) 5(2.35) 0.229 非致死性MI 2(0.56) 0(0) 2(0.94) 0.245 ST 0(0) 0(0) 0(0) >0.999 MACE 39(10.99) 16(11.27) 23(10.80) 0.884 ST:支架内血栓。 表 5 多因素Cox比例风险模型
Table 5. Multivariate Cox proportional hazards model
因素 OR(95%CI) P 单纯DCB(vs DCB+DES) 1.335(0.533~3.344) 0.537 男性 1.479(0.544~4.025) 0.443 年龄>65岁 1.083(0.443~2.649) 0.862 高血压 1.530(0.633~3.699) 0.345 糖尿病 1.075(0.461~2.511) 0.866 高脂血症 0.959(0.430~2.142) 0.919 多支病变 1.289(0.483~3.443) 0.612 吸烟 0.493(0.181~1.343) 0.167 冠心病家族史 1.009(0.413~2.469) 0.984 卒中史 1.530(0.529~4.420) 0.432 既往MI 0.502(0.145~1.739) 0.277 既往PCI 2.176(0.825~5.735) 0.116 LVEF>50% 1.642(0.354~7.606) 0.526 靶血管(LCX vs LAD) 0.458(0.120~1.753) 0.254 靶血管(RCA vs LAD) 0.849(0.349~2.064) 0.718 CTO病变 1.069(0.441~2.589) 0.883 钙化 1.045(0.414~2.639) 0.926 病变长度>45 mm 2.716(1.046~7.052) 0.040 参考血管直径>2.5 mm 0.778(0.326~1.857) 0.572 -
[1] Lozano I, Capin E, de la Hera JM, et al. Diffuse Coronary Artery Disease Not Amenable to Revascularization: Long-term Prognosis[J]. Rev Esp Cardiol(Engl Ed), 2015, 68(7): 631-633. doi: 10.1016/j.recesp.2015.02.013
[2] Palmerini T, Benedetto U, Biondi-Zoccai G, et al. Long-Term Safety of Drug-Eluting and Bare-Metal Stents: Evidence From a Comprehensive Network Meta-Analysis[J]. J Am Coll Cardiol, 2015, 65(23): 2496-2507. doi: 10.1016/j.jacc.2015.04.017
[3] Otsuka F, Vorpahl M, Nakano M, et al. Pathology of second-generation everolimus-eluting stents versus first-generation sirolimus-and paclitaxel-eluting stents in humans[J]. Circulation, 2014, 129(2): 211-223. doi: 10.1161/CIRCULATIONAHA.113.001790
[4] Lee CW, Park DW, Lee BK, et al. Predictors of restenosis after placement of drug-eluting stents in one or more coronary arteries[J]. Am J Cardiol, 2006, 97(4): 506-511. doi: 10.1016/j.amjcard.2005.09.084
[5] D'Ascenzo F, Bollati M, Clementi F, et al. Incidence and predictors of coronary stent thrombosis: evidence from an international collaborative meta-analysis including 30 studies, 221, 066 patients, and 4276 thromboses[J]. Int J Cardiol, 2013, 167(2): 575-584. doi: 10.1016/j.ijcard.2012.01.080
[6] Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization[J]. Kardiol Pol, 2018, 76(12): 1585-1664. doi: 10.5603/KP.2018.0228
[7] 杨新越, 潘亮, 郑悠阳, 等. 药物涂层球囊在冠状动脉原位病变中的应用现状[J]. 临床心血管病杂志, 2021, 37(8): 695-699. https://www.cnki.com.cn/Article/CJFDTOTAL-LCXB202108003.htm
[8] Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS[J]. Eur Heart J, 2018, 39(3): 213-260. doi: 10.1093/eurheartj/ehx419
[9] 林珑, 刘冠男, 高丽霓, 等. 经皮冠状动脉介入术后主要不良心脏事件危险因素研究进展[J]. 临床急诊杂志, 2020, 21(11), 918-922. https://www.cnki.com.cn/Article/CJFDTOTAL-ZZLC202011015.htm
[10] Madhavan MV, Redfors B, Ali ZA, et al. Long-Term Outcomes After Revascularization for Stable Ischemic Heart Disease: An Individual Patient-Level Pooled Analysis of 19 Randomized Coronary Stent Trials[J]. Circ Cardiovasc Interv, 2020, 13(4): e008565. doi: 10.1161/CIRCINTERVENTIONS.119.008565
[11] Yerasi C, Case BC, Forrestal BJ, et al. Drug-Coated Balloon for De Novo Coronary Artery Disease: JACC State-of-the-Art Review[J]. J Am Coll Cardiol, 2020, 75(9): 1061-1073. doi: 10.1016/j.jacc.2019.12.046
[12] Jeger RV, Eccleshall S, Wan Ahmad WA, et al. Drug-Coated Balloons for Coronary Artery Disease: Third Report of the International DCB Consensus Group[J]. JACC Cardiovasc Interv, 2020, 13(12): 1391-1402. doi: 10.1016/j.jcin.2020.02.043
[13] Meneguz-Moreno RA, Ribamar Costa J Jr, Abizaid A. Drug-Coated Balloons: Hope or Hot Air: Update on the Role of Coronary DCB[J]. Curr Cardiol Rep, 2018, 20(10): 100. doi: 10.1007/s11886-018-1025-4
[14] Picard F, Doucet S, Asgar AW. Contemporary use of drug-coated balloons in coronary artery disease: Where are we now?[J]. Arch Cardiovasc Dis, 2017, 110(4): 259-272. doi: 10.1016/j.acvd.2017.01.005
[15] Wöhrle J, Scheller B, Seeger J, et al. Impact of Diabetes on Outcome With Drug-Coated Balloons Versus Drug-Eluting Stents: The BASKET-SMALL 2 Trial[J]. JACC Cardiovasc Interv, 2021, 14(16): 1789-1798. doi: 10.1016/j.jcin.2021.06.025
[16] Costopoulos C, Latib A, Naganuma T, et al. The role of drug-eluting balloons alone or in combination with drug-eluting stents in the treatment of de novo diffuse coronary disease[J]. JACC Cardiovasc Interv, 2013, 6(11): 1153-1159. doi: 10.1016/j.jcin.2013.07.005
[17] Im E, Kim BK, Ko YG, et al. Comparison of 3-year clinical outcomes between ResoluteTM zotarolimus-and sirolimus-eluting stents for long coronary artery stenosis[J]. J Interv Cardiol, 2013, 26(4): 378-383. doi: 10.1111/joic.12047
[18] Köln PJ, Scheller B, Liew HB, et al. Treatment of chronic total occlusions in native coronary arteries by drug-coated balloons without stenting-A feasibility and safety study[J]. Int J Cardiol, 2016, 225: 262-267. doi: 10.1016/j.ijcard.2016.09.105
[19] 韩旭飞, 刘恒道, 邢军辉, 等. 单纯使用药物涂层球囊治疗冠状动脉慢性完全闭塞性病变的临床疗效分析[J]. 临床心血管病杂志, 2021, 37(7): 604-609. https://www.cnki.com.cn/Article/CJFDTOTAL-LCXB202107004.htm
[20] Jeger RV, Farah A, Ohlow MA, et al. Long-term efficacy and safety of drug-coated balloons versus drug-eluting stents for small coronary artery disease(BASKET-SMALL 2): 3-year follow-up of a randomised, non-inferiority trial[J]. Lancet, 2020, 396(10261): 1504-1510.
[21] Her AY, Shin ES, Chung JH, et al., Plaque modification and stabilization after paclitaxel-coated balloon treatment for de novo coronary lesions[J]. Heart Vessels, 2019, 34(7): 1113-1121.
-




 下载:
下载: