Outcomes of drug-coated balloon versus drug-eluting stent in the de novo lesions of saphenous vein grafts
-
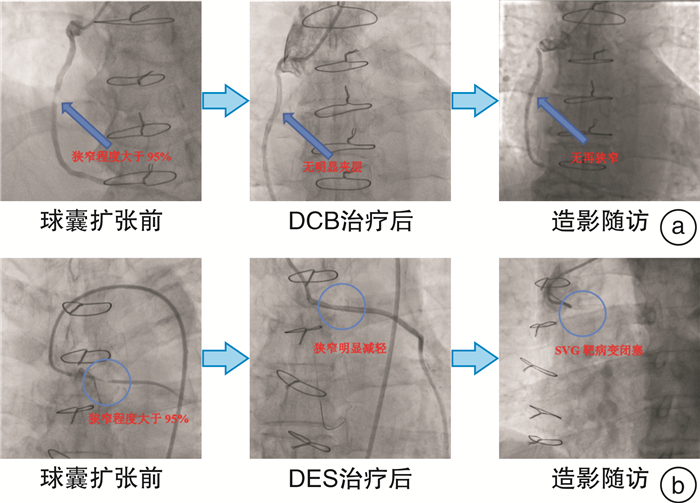
摘要: 目的探讨药物涂层球囊(DCB)治疗冠心病患者静脉桥血管(SVG)原位病变的有效性和安全性。方法回顾性连续纳入2018年1月—2020年12月在接受DCB或药物洗脱支架(DES)治疗冠状动脉旁路移植(CABG)术后SVG原位病变的冠心病患者,分为DCB组和DES组。分析两组的基线和病变特征,主要终点是靶病变再狭窄,次要终点是靶病变血运重建(TLR)和不良心血管事件(MACE),后者包括靶病变再狭窄、心源性死亡、急性心肌梗死以及靶血管血运重建(TVR)。结果本研究共纳入54例患者,其中DCB组31例(33处病变),DES组23例(24处病变)。两组的基线、病变特征均无统计学差异。DCB组中位随访时间为17.0个月,DES组中位随访时间20.0个月(P>0.05)。结果显示,DCB组与DES组的靶病变再狭窄发生率无统计学差异(12.9% vs 21.7%,log-rank P=0.470),两组的TLR及MACE事件发生率也相似(TLR:12.9% vs 4.3%,log-rank P=0.168;MACE:16.1% vs 26.1%,log-rank P=0.663)。结论同DES相比,DCB在冠心病患者SVG原位病变中的应用是有效、安全的。Abstract: ObjectiveThis study aims to explore the outcomes of the drug-coated balloon(DCB) in the de novo lesions of saphenous vein grafts(SVG).MethodsThis observational study analyzed the patients treated with DCB or drug-eluting stent(DES) in SVG de novo lesions from January 2018 to December 2020. Restenosis was the primary endpoint, and target lesion revascularization(TLR) and major adverse cardiac events(MACE), including all examples of restenosis, cardiac death, target vessel revascularization, and myocardial infarction as the secondary outcomes.ResultsWe enrolled 31 patients with DCB and 23 with DES(57 lesions in total). Baseline clinical data, lesion characteristics, and procedural characteristics were all similar between the two groups. The rate of restenosis was 12.9% versus 21.7%(log-rank P=0.470) between DCB and DES group. No significant differences were observed in the rates of MACE(16.1% vs 26.1%, log-rank P=0.663) and TLR(12.9% vs 4.3%, log-rank P=0.168) during the clinical follow-up.ConclusionDCB is efficient and safe in treating SVG de novo lesions compared with new-generation DES.
-

-
表 1 DCB组与DES组临床基线资料比较
Table 1. General data
例(%), X±S, M(P25, P75) 项目 DCB组(31例) DES组(23例) P值 年龄/岁 66.7±9.4 64.0±9.7 0.299 男性 21(67.7) 16(69.6) 0.887 糖尿病 11(35.5) 9(39.1) 0.784 高血压 24(77.4) 21(91.3) 0.273 高脂血症* 18(58.1) 11(47.8) 0.456 临床表现 0.463 不稳定型心绞痛 23(74.2) 18(78.3) 非ST段抬高型心肌梗死 7(22.6) 5(21.7) ST段抬高型心肌梗死 1(3.2) 0(0) 吸烟史 4(12.9) 5(9.3) 0.274 家族冠心病史 4(12.9) 7(30.4) 0.173 既往心肌梗死史 13(41.9) 14(60.9) 0.169 既往PCI史 5(16.1) 5(21.7) 0.211 冠脉血管病变 0.641 1支病变 1(3.2) 0(0) 2支病变 5(16.1) 3(13.0) 3支病变 25(80.6) 20(87.0) 桥龄/年 9.16±5.1 8.65±5.3 0.722 左室射血分数/% 60(55,63) 57(53,60) 0.630 肌酐/(μmol·L-1) 81.90±19.4 80.65±25.26 0.827 *高脂血症:总胆固醇>5.18 mmol/L或LDL>2.37 mmol/L或甘油三酯>1.7 mmol/L。 表 2 两组介入手术相关资料比较
Table 2. Related interventional data between the two groups
M(P25, P75) 项目 DCB组(31例) DES组(23例) P值 病变/处 33 24 - 直径狭窄率 > 70%的病变/处(%) 21(63.6) 13(54.2) 0.472 桥血管狭窄部位/处(%) 0.326 主动脉吻合口 3(9.1) 6(25.0) 冠脉吻合口 2(6.1) 4(16.7) 桥血管近段 8(24.2) 4(16.7) 桥血管中段 15(45.5) 9(37.5) 桥血管远段 5(15.2) 1(4.2) 球囊预扩张后的血流分级/处(%) 0.710 0级 0(0) 0(0) 1级 1(3.0) 3(12.5) 2级 3(9.1) 1(4.2) 3级 29(87.9) 20(83.3) 术后慢血流/无复流/例(%) 0(0) 0(0) - 预扩张 半顺应性球囊直径/mm 2.0(2.0,2.5) 2.0(2.0,2.5) 0.774 半顺应性球囊长度/mm 15.0(15.0,20.0) 15.0(15.0,20.0) 0.402 切割球囊/例(%) 9(29.0) 1(4.3) 0.031 双导丝球囊/例(%) 12(38.7) 1(4.3) 0.003 DCB或DES长度/mm 20.0(17.0,30.0) 23.0(16.0,28.0) 0.536 DCB或DES直径/mm 3.0(2.65,3.50) 3.375(2.5,3.85) 0.503 表 3 两组随访结果比较
Table 3. Comparison of follow-up results between the two groups
例(%), M(P25, P75) 项目 DCB组(31例) DES组(23例) P值 造影随访例数 28(90.3) 21(91.3) 0.788 临床随访时间/月 17.0(8.8,32.3) 20.0(13.5,25.3) 0.409 心源性死亡 0(0) 1(4.3) 0.240 心肌梗死 0(0) 0(0) - TLR 4(12.9) 1(4.3) 0.168 TVR 5(16.1) 1(4.3) 0.116 MACE 5(16.1) 6(26.1) 0.663 靶病变再狭窄 4(12.9) 5(21.7) 0.470 靶血管闭塞 0(0) 4(17.4) 0.027 表 4 两组危险因素分析
Table 4. Analysis of risk factors in the two groups
X±S 危险因素 DCB组(31例) DES组(23例) 术前 随访 P值 术前 随访 P值 LDL/(mmol·L-1) 2.44±1.04 1.72±0.52 0.021 1.63±0.54 1.77±0.60 0.376 空腹血糖/(mmol·L-1) 6.11±3.03 6.21±1.88 0.884 6.30±1.94 6.23±0.77 0.892 糖化血红蛋白/% 6.63±1.43 6.60±1.55 0.770 6.11±0.76 6.23±0.77 0.429 平均收缩压/mmHg 134.12±25.12 133.31±14.31 0.736 135.90±17.24 132.55±12.80 0.669 平均舒张压/mmHg 76.60±6.09 75.27±9.10 0.419 77.36±7.95 73.36±10.63 0.365 抽烟人数/例(%) 4 (12.9) 0 (0) 0.039 5 (21.7) 0 (0) 0.049 -
[1] Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization[J]. Eur Heart J, 2019, 40(2): 87-165. doi: 10.1093/eurheartj/ehy394
[2] Ren Y, Song B, Li J, et al. Underlying mechanisms of saphenous vein graft stenosis after coronary artery bypass caused by clipping of the side branches: an experimental study[J]. J Thorac Dis, 2022, 14(4): 1088-1098. doi: 10.21037/jtd-22-235
[3] 韩旭飞, 刘恒道, 邢军辉, 等. 单纯使用药物涂层球囊治疗冠状动脉慢性完全闭塞性病变的临床疗效分析[J]. 临床心血管病杂志, 2021, 37(7): 604-609. doi: 10.13201/j.issn.1001-1439.2021.07.004
[4] Beerkens FJ, Claessen BE, Mahan M, et al. Contemporary coronary artery bypass graft surgery and subsequent percutaneous revascularization[J]. Nat Rev Cardiol, 2022, 19(3): 195-208. doi: 10.1038/s41569-021-00612-6
[5] Colleran R, Kufner S, Mehilli J, et al. Efficacy over time with drug-eluting stents in saphenous vein graft lesions[J]. J Am Coll Cardiol, 2018, 71(18): 1973-1982. doi: 10.1016/j.jacc.2018.03.456
[6] Brilakis ES, Lichtenwalter C, Abdel-karim AR, et al. Continued benefit from paclitaxel-eluting compared with bare-metal stent implantation in saphenous vein graft lesions during long-term follow-up of the SOS(Stenting of Saphenous Vein Grafts)trial[J]. JACC Cardiovasc Interv, 2011, 4(2): 176-182. doi: 10.1016/j.jcin.2010.10.003
[7] Brilakis ES, Edson R, Bhatt DL, et al. Drug-eluting stents versus bare-metal stents in saphenous vein grafts: a double-blind, randomised trial[J]. Lancet, 2018, 391(10134): 1997-2007. doi: 10.1016/S0140-6736(18)30801-8
[8] 杨新越, 潘亮, 郑悠阳, 等. 药物涂层球囊在冠状动脉原位病变中的应用现状[J]. 临床心血管病杂志, 2021, 37(8): 695-699. doi: 10.13201/j.issn.1001-1439.2021.08.003
[9] 桑震池, 李敏, 刘乐琳, 等. 药物涂层球囊在NSTE-ACS患者中的安全性和有效性比较: 9个月随访结果分析[J]. 临床心血管病杂志, 2020, 36(1): 40-43. https://www.cnki.com.cn/Article/CJFDTOTAL-LCXB202001009.htm
[10] Jeger RV, Eccleshall S, Wan Ahmad WA, et al. Drug-coated balloons for coronary artery disease: third report of the international dcb consensus group[J]. JACC Cardiovasc Interv, 2020, 13(12): 1391-1402. doi: 10.1016/j.jcin.2020.02.043
[11] Pan L, Lu W, Han Z, et al. Clinical outcomes of drug-coated balloon in coronary lesions: a real-world, all-comers study[J]. Clin Res Cardiol, 2022, 111(7): 732-741. doi: 10.1007/s00392-021-01895-y
[12] Holmes DR Jr, Vlietstra RE, Smith HC, et al. Restenosis after percutaneous transluminal coronary angioplasty(PTCA): a report from the PTCA Registry of the National Heart, Lung, and Blood Institute[J]. Am J Cardiol, 1984, 53(12): 77C-81C. doi: 10.1016/0002-9149(84)90752-5
[13] Jeger RV, Möbius-Winkler S. Stents in saphenous vein grafts[J]. Lancet, 2018, 391(10134): 1967-1968. doi: 10.1016/S0140-6736(18)30937-1
[14] Sarjeant JM, Rabinovitch M. Understanding and treating vein graft atherosclerosis[J]. Cardiovasc Pathol, 2002, 11(5): 263-271. doi: 10.1016/S1054-8807(02)00125-4
[15] Yerasi C, Case BC, Forrestal BJ, et al. Drug-coated balloon for de novo coronary artery disease: JACC State-of-the-Art Review[J]. J Am Coll Cardiol, 2020, 75(9): 1061-1073. doi: 10.1016/j.jacc.2019.12.046
[16] Ang H, Lin J, Huang YY, et al. Drug-coated balloons: technologies and clinical applications[J]. Curr Pharm Des, 2018, 24(4): 381-396. doi: 10.2174/1381612824666171227221305
[17] Jeger RV, Farah A, Ohlow MA, et al. Drug-coated balloons for small coronary artery disease(BASKET-SMALL 2): an open-label randomised non-inferiority trial[J]. Lancet, 2018, 392(10150): 849-856. doi: 10.1016/S0140-6736(18)31719-7
[18] Wöhrle J, Scheller B, Seeger J, et al. Impact of diabetes on outcome with drug-coated balloons versus drug-eluting stents: The BASKET-SMALL 2 Trial[J]. JACC Cardiovasc Interv, 2021, 14(16): 1789-1798. doi: 10.1016/j.jcin.2021.06.025
[19] Cortese B, Silva Orrego P, Agostoni P, et al. Effect of drug-coated balloons in native coronary artery disease left with a dissection[J]. JACC Cardiovasc Interv, 2015, 8(15): 2003-2009. doi: 10.1016/j.jcin.2015.08.029
[20] Mehilli J, Pache J, Abdel-Wahab M, et al. Drug-eluting versus bare-metal stents in saphenous vein graft lesions(ISAR-CABG): a randomised controlled superiority trial[J]. Lancet, 2011, 378(9796): 1071-1078. doi: 10.1016/S0140-6736(11)61255-5
[21] Linni K, Ugurluoglu A, Aspalter M, et al. Paclitaxel-coated versus plain balloon angioplasty in the treatment of infrainguinal vein bypass stenosis[J]. J Vasc Surg, 2016, 63(2): 391-398. doi: 10.1016/j.jvs.2015.08.081
[22] Björkman P, Kokkonen T, Albäck A, et al. Drug-coated versus plain balloon angioplasty in bypass vein grafts(the DRECOREST Ⅰ-Study)[J]. Ann Vasc Surg, 2019, 55: 36-44. doi: 10.1016/j.avsg.2018.04.042
[23] Wang JH, Liu W, Du X, et al. Long term outcomes of saphaneous vein graft intervention in elderly patients with prior coronary artery bypass graft[J]. J Geriatr Cardiol, 2014, 11(1): 26-31.
[24] Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions[J]. Catheter Cardiovasc Interv, 2012, 79(3): 453-495. doi: 10.1002/ccd.23438
[25] Shoaib A, Kinnaird T, Curzen N, et al. Outcomes following percutaneous coronary intervention in saphenous vein grafts with and without embolic protection devices[J]. JACC Cardiovasc Interv, 2019, 12(22): 2286-2295. doi: 10.1016/j.jcin.2019.08.037
[26] Vos NS, Fagel ND, Amoroso G, et al. Paclitaxel-coated balloon angioplasty versus drug-eluting stent in acute myocardial infarction: the REVELATION Randomized Trial[J]. JACC Cardiovasc Interv, 2019, 12(17): 1691-1699. doi: 10.1016/j.jcin.2019.04.016
[27] Tian J, Tang YD, Qiao S, et al. Two-year follow-up of a randomized multicenter study comparing a drug-coated balloon with a drug-eluting stent in native small coronary vessels: The RESTORE Small Vessel Disease China trial[J]. Catheter Cardiovasc Interv, 2020, 95 Suppl 1: 587-597.
-




 下载:
下载: