Long-term prognostic value of whole blood cell-derived inflammatory markers in patients with acute heart failure
-
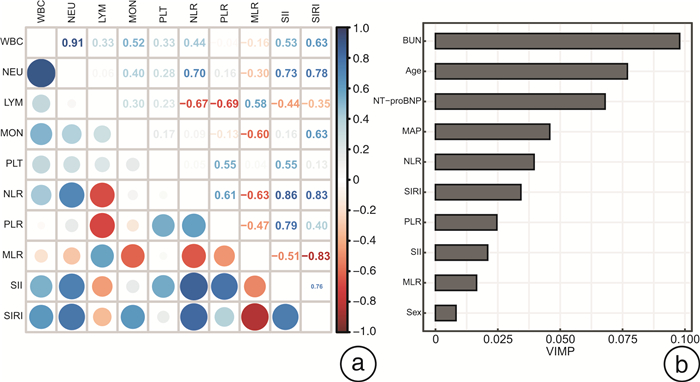
摘要: 目的 本研究旨在评估全血细胞衍生的炎症标志物[包括中性粒细胞与淋巴细胞比值(NLR)、血小板与淋巴细胞比值(PLR)、单核细胞与淋巴细胞比值(MLR)、全身免疫炎症指数(SII)和全身炎症反应指数(SIRI)]与急性心力衰竭(AHF)患者全因死亡的关系。方法 本研究是一项前瞻性队列研究,2012年4月—2016年5月连续入组538例AHF患者,并随访至2019年3月。通过ROC曲线确定炎症标志物预测AHF患者全因死亡的最佳临界值并进行分组。使用Kaplan-Meier法绘制生存曲线,log-rank检验比较组间生存有无差异。多因素Cox回归评估炎症标志物与AHF全因死亡的关系。AUC、综合判别改进指数和连续净重分类改进指数评估炎症生物标志对AHF患者的基础预测模型改善情况。限制性立方样条回归和分段线性回归探究全血细胞衍生的炎症标志物与AHF全因死亡的阈值效应。最后,采用随机生存森林模型估计衍生的炎症标志物在AHF全因死亡风险中的相对重要性。结果 中位随访34个月,全因死亡227例(42.2%)。多变量逐步回归显示,年龄、性别、平均动脉压、尿素氮、和N-末端脑钠肽前体(NT-proBNP)是AHF患者全因死亡的独立危险因素。校正上述变量后,NLR(P=0.011)、MLR(P=0.015)、SII(P=0.026)和SIRI(P=0.017)与AHF患者全因死亡风险独立相关,其中NLR和MLR可显著改变模型对AHF全因死亡的预测能力。样条回归结果显示,NLR、MLR、SII、SIRI与AHF全因死亡风险呈线性关系,血小板(PLT)和PLR与AHF预后呈非线性关系,其阈值拐点分别为159.676×109/L和111.585。随机生存森林模型表明,NLR是5种全血细胞衍生的炎症标志物中最重要的预测因子。结论 炎症生物标志物与AHF患者全因死亡风险有关,其中NLR是衍生炎症标志物中最重要的预测因子,可显著改善模型的预测能力,而PLR与AHF全因死亡风险存在非线性“U”型关系。Abstract: Objective Systemic inflammation is associated with poor prognosis in acute heart failure (AHF). This study was to evaluate the association of whole blood cell-derived inflammatory markers [including neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), monocyte-to-lymphocyte ratio (MLR), systemic immune-inflammation index (SII), and systemic inflammation response index (SIRI)] with all-cause mortality in patients with AHF.Methods This study was a prospective cohort study with 538 consecutive patients with AHF enrolled from April 2012 to May 2016, and followed up until March 2019. The optimal threshold of inflammatory markers to predict all-cause mortality in AHF patients was determined by ROC curves and the participants were then divided into separate groups based on this threshold.Survival curves were plotted using the Kaplan-Meier method, and log-rank tests were performed to compare any differences in all-cause mortality between inflammatory marker groups. Multifactorial Cox regression was performed to assess the association between inflammatory markers and all-cause mortality in AHF. We usedtheAUC, integrated discrimination improvement (IDI), and continuous net reclassification improvement(c-NRI) to assess the improvement of the underlying predictive model of inflammatory biomarkers in AHF patients. Restricted cubic spline regression and segmented linear regression were used to explore the threshold effects of inflammatory markers and all-cause mortality in AHF. Finally, a randomized survival forest model was used to estimate the relative importance of each inflammatory marker in the risk of all-cause mortality in AHF.Results At a median follow-up of 34 months, there were 227 all-cause deaths(42.2%). Multivariate stepwise regression showed that age(P < 0.001), gender(P=0.003), mean arterial pressure(P < 0.001), urea nitrogen(P=0.005), and NT-proBNP(P < 0.001) were independent risk factors for all-cause mortality in patients with AHF. After adjustment for the above variables, NLR(P=0.011), MLR(P=0.015), SII(P=0.026), and SIRI(P=0.017) were all independently associated with the risk of all-cause mortality in AHF patients. Notably, NLR and MLR significantly altering the predictive power of our model for all-cause mortality in AHF. Restricted cubic spline regression results showed that NLR, MLR, SII, and SIRI were linearly related to the risk of all-cause death in AHF, and platelets(PLT) and PLR were nonlinearly related to AHF prognosis with threshold inflection points of 159.676×109/L and 111.585, respectively. A random survival forest model indicated that NLR was the most important predictor of the five all-cause cell-derived inflammatory markers.Conclusion Inflammatory biomarkers were associated with all-cause mortality risk in AHF patients, with NLR being the most important predictor among the derived inflammatory markers and significantly improving the predictive power of the model, while PLR had a non-linear "U" shaped relationship with all-cause mortality risk in AHF.
-
Key words:
- acute heart failure /
- inflammatory biomarkers /
- all-cause mortality
-

-
表 1 AHF患者基线特征
Table 1. Baseline characteristics of patients with AHF
X±S, M(P25, P75) 变量 生存组(311例) 死亡组(227例) P值 年龄/岁 59.39±28.25 65.23 ±15.16 0.005 男性/例(%) 228(73.3) 129(56.8) < 0.001 高血压/例(%) 166(53.4) 109(48.0) 0.254 糖尿病/例(%) 74(23.8) 57(25.1) 0.803 缺血性心衰/例(%) 121(38.9) 83(36.6) 0.643 NYHA/例(%) 0.009 Ⅱ 65(20.9) 26(11.5) Ⅲ 164(52.7) 125(55.1) Ⅳ 82(26.4) 76(33.5) 心率/(次·min-1) 80.13±15.31 77.48±15.86 0.051 收缩压/mmHg 128.71±23.56 123.45±19.49 < 0.001 舒张压/mmHg 80.50±16.43 75.64±12.23 < 0.001 平均动脉压/mmHg 96.57±16.93 91.58±12.94 < 0.001 WBC/(×109·L-1) 6.54(5.30,8.00) 6.80(5.29,9.00) 0.196 NEU/(×109·L-1) 4.19(3.13,5.44) 4.38(3.40,6.64) 0.026 LYM/(×109·L-1) 1.65(1.24,2.12) 1.48(1.06,1.97) 0.002 MON/(×109·L-1) 0.43(0.33,0.58) 0.48(0.34,0.62) 0.143 血红蛋白/(g·L-1) 136.00(123.00,149.00) 129.00(116.00,144.00) < 0.001 PLT/(×109·L-1) 167.00(134.00,205.00) 154.00(116.00,209.00) 0.040 NLR 2.40(1.64,4.04) 3.05(2.14,5.21) < 0.001 PLR 97.73(75.31,134.93) 105.75(75.78,146.30) 0.178 MLR 0.26(0.19,0.40) 0.32(0.22,0.45) 0.001 SII/(×109·L-1) 398.01(257.22,689.35) 465.68(315.12,776.62) 0.015 SIRI/(×109·L-1) 1.08(0.64,2.05) 1.46(0.86,2.55) < 0.001 总胆固醇/(mmol·L-1) 4.03±0.99 3.91±1.11 0.199 甘油三酯/(mmol·L-1) 1.33±0.71 1.14±0.56 < 0.001 HDL-C/(mmol·L-1) 0.98±0.27 0.99±0.32 0.542 LDL-C/(mmol·L-1) 2.59±0.78 2.52±0.95 0.369 血糖/(mmol·L-1) 5.44±1.77 5.70±2.41 0.150 血尿素氮/(mmol·L-1) 16.01±11.25 19.99±11.98 < 0.001 血肌酐/(μmoI·L-1) 84.10(70.80,103.25) 94.90(75.40,119.95) < 0.001 eGFR/[mL·min-1·(1.73 m2)-1] 78.00±24.85 65.49±26.35 < 0.001 肌钙蛋白T/(ng·mL-1) 0.35(0.05,31.74) 0.05(0.05,19.40) 0.008 sST2/(ng·mL-1) 32.08(18.07,51.04) 38.52(23.18,67.97) 0.001 NT-proBNP/(ng·L-1) 1791.00(1087.00,4643.50) 2777.00(1590.00,7250.00) < 0.001 左室射血分数/% 41.07±14.14 43.54±14.81 0.050 左室舒张末期内径/mm 62.12±11.67 61.07±12.72 0.323 左室收缩末期内径/mm 49.71±13.30 48.09±14.47 0.180 BMI/(kg·m-2) 24.44±4.33 23.96±4.80 0.228 利尿剂/例(%) 294(94.5) 216(95.2) 0.902 醛固酮受体拮抗剂/例(%) 279(89.7) 200(88.1) 0.654 ACEI/ARB/例(%) 248(79.7) 169(74.4) 0.178 β受体阻滞剂/例(%) 247(79.4) 180(79.3) 0.967 注:NYHA:纽约心功能分级;HDL-C:高密度脂蛋白胆固醇;LDL-C:低密度脂蛋白胆固醇;sST2:可溶性生长刺激表达基因2蛋白;BMI:体重指数;ACEI:血管紧张素转化酶抑制剂;ARB:血管紧张素受体拮抗剂。 表 2 全血细胞衍生的炎症标志物预测AHF患者3年全因死亡的曲线下面积和最佳临界值
Table 2. Area under the curve and optimal thresholds for whole blood cell-derived inflammatory markers to predict all-cause mortality at 3 years in patients with AHF
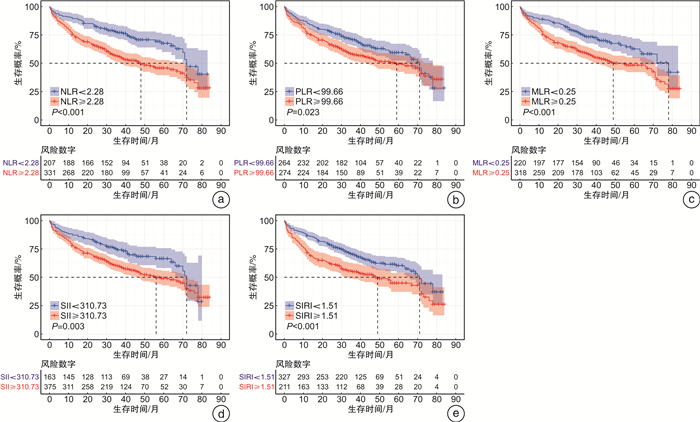
炎症标志物 AUC(95%CI) 最佳临界值 敏感性 特异性 NLR 0.637(0.584~0.690) 2.28 0.751 0.480 PLR 0.549(0.493~0.606) 99.66 0.595 0.556 MLR 0.587(0.532~0.643) 0.25 0.684 0.476 SII 0.571(0.515~0.626) 310.73 0.782 0.360 SIRI 0.604(0.549~0.659) 1.51 0.548 0.631 表 3 多因素Cox回归分析评估炎症生物标志物与AHF全因死亡风险的关系
Table 3. Multifactorial Cox regression analysis to assess the association between inflammatory biomarkers and the risk of all-cause mortality in AHF
变量 分类变量 Log2转换后连续变量 HR(95%CI) P值 HR(95%CI) P值 NLR 1.483(1.095~2.008) 0.011 1.266(1.094~1.466) 0.002 PLR 1.254(0.959~1.640) 0.098 1.061(0.894~1.258) 0.497 MLR 1.431(1.071~1.912) 0.015 1.208(1.012~1.441) 0.036 SII 1.424(1.043~1.943) 0.026 1.157(1.023~1.309) 0.020 SIRI 1.387(1.061~1.814) 0.017 1.213(1.077~1.366) 0.001 表 4 基础模型分别加入全血细胞衍生的炎症标志物对3年预后模型的改善情况
Table 4. Improvement of 3-year prognostic model by adding whole blood cell-derived inflammatory markers to the base model separately
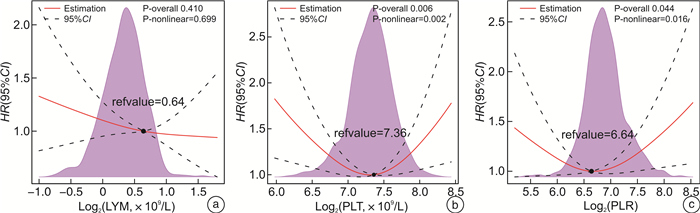
变量 AUC IDI c-NRI 基础模型 0.738(0.690~0.787) - - +NLR 0.750(P=0.111) 0.013(P=0.044) 0.227(P=0.032) +PLR 0.745(P=0.167) 0.008(P=0.140) 0.151(P=0.112) +MLR 0.741(P=0.680) 0.008(P=0.132) 0.152(P=0.044) +SII 0.744(P=0.363) 0.009(P=0.100) 0.153(P=0.064) +SIRI 0.741(P=0.703) 0.008(P=0.168) 0.152(P=0.068) 表 5 阈值效应分析PLT、PLR与AHF患者全因死亡的关系
Table 5. Threshold effect analysis of PLT, and PLR in relation to all-cause mortality in patients with AHF
变量 拐点 Log2转换 分组 HR(95%CI) P值 似然比检验P值 PLT 159.676×109/L 7.319×109/L < 7.319×109/L 0.643(0.419~0.987) 0.043 < 0.001 ≥7.319×109/L 1.968(1.160~3.340) 0.012 PLR 111.585 6.802 < 6.802 0.721(0.506~1.028) 0.071 < 0.001 ≥6.802 1.115(0.786~1.581) 0.542 -
[1] Mann DL. The emerging role of innate immunity in the heart and vascular system: for whom the cell tolls[J]. Circ Res, 2011, 108(9): 1133-1145. doi: 10.1161/CIRCRESAHA.110.226936
[2] Murphy SP, Kakkar R, McCarthy CP, et al. Inflammation in Heart Failure: JACC State-of-the-Art Review[J]. J Am Coll Cardiol, 2020, 75(11): 1324-1340.
[3] 廖玉华, 杨杰孚, 张健, 等. 舒张性心力衰竭诊断和治疗专家共识[J]. 临床心血管病杂志, 2020, 36(1): 1-10. https://www.cnki.com.cn/Article/CJFDTOTAL-LCXB202001001.htm
[4] 中国心衰中心联盟. 舒张性心力衰竭早期防治专家建议[J]. 临床心血管病杂志, 2021, 37(1): 1-6. https://www.cnki.com.cn/Article/CJFDTOTAL-LCXB202101001.htm
[5] Passino C, Barison A, Vergaro G, et al. Markers of fibrosis, inflammation, and remodeling pathways in heart failure[J]. Clin Chim Acta, 2015, 443: 29-38. doi: 10.1016/j.cca.2014.09.006
[6] Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association[J]. Circulation, 2003, 107(3): 499-511. doi: 10.1161/01.CIR.0000052939.59093.45
[7] Greene SJ, Harinstein ME, Vaduganathan M, et al. Prognostic value of monocyte count in patients hospitalized for heart failure with reduced ejection fraction(from the EVEREST Trial)[J]. Am J Cardiol, 2012, 110(11): 1657-1662. doi: 10.1016/j.amjcard.2012.07.035
[8] Shantsila E, Bialiuk N, Navitski D, et al. Blood leukocytes in heart failure with preserved ejection fraction: impact on prognosis[J]. Int J Cardiol, 2012, 155(2): 337-338. doi: 10.1016/j.ijcard.2011.12.048
[9] Milo-Cotter O, Teerlink JR, Metra M, et al. Low lymphocyte ratio as a novel prognostic factor in acute heart failure: results from the Pre-RELAX-AHF study[J]. Cardiology, 2010, 117(3): 190-196. doi: 10.1159/000321416
[10] Carubelli V, Bonadei I, Castrini AI, et al. Prognostic value of the absolute lymphocyte count in patients admitted for acute heart failure[J]. J Cardiovasc Med(Hagerstown), 2017, 18(11): 859-865. doi: 10.2459/JCM.0000000000000428
[11] Kyne L, Hausdorff JM, Knight E, et al. Neutrophilia and congestive heart failure after acute myocardial infarction[J]. Am Heart J, 2000, 139(1 Pt 1): 94-100.
[12] Song PS, Ahn KT, Jeong JO, et al. Association of baseline platelet count with all-cause mortality after acute myocardial infarction[J]. Eur Heart J Acute Cardiovasc Care, 2020.
[13] Melchio R, Rinaldi G, Testa E, et al. Red cell distribution width predicts mid-term prognosis in patients hospitalized with acute heart failure: the RDW in Acute Heart Failure(RE-AHF)study[J]. Intern Emerg Med, 2019, 14(2): 239-247. doi: 10.1007/s11739-018-1958-z
[14] O'Connor CM, Starling RC, Hernandez AF, et al. Effect of nesiritide in patients with acute decompensated heart failure[J]. N Engl J Med, 2011, 365(1): 32-43. doi: 10.1056/NEJMoa1100171
[15] 陈娟, 石志, 娄慧娟, 等. 系统免疫-炎症指数对初诊多发性骨髓瘤患者预后的评估价值[J]. 临床血液学杂志, 2022, 35(3): 180-186. https://www.cnki.com.cn/Article/CJFDTOTAL-LCXZ202203006.htm
[16] Curran FM, Bhalraam U, Mohan M, et al. Neutrophil-to-lymphocyte ratio and outcomes in patients with new-onset or worsening heart failure with reduced and preserved ejection fraction[J]. ESC Heart Fail, 2021, 8(4): 3168-3179. doi: 10.1002/ehf2.13424
[17] Angkananard T, Inthanoo T, Sricholwattana S, et al. The Predictive role of Neutrophil-to-Lymphocyte Ratio(NLR)and Mean Platelet Volume-to-Lymphocyte Ratio(MPVLR)for Cardiovascular Events in Adult Patients with Acute Heart Failure[J]. Mediators Inflamm, 2021, 2021: 6889733.
[18] Heidarpour M, Bashiri S, Vakhshoori M, et al. The association between platelet-to-lymphocyte ratio with mortality among patients suffering from acute decompensated heart failure[J]. BMC Cardiovasc Disord, 2021, 21(1): 454. doi: 10.1186/s12872-021-02260-7
[19] Tang Y, Zeng X, Feng Y, et al. Association of Systemic Immune-Inflammation Index With Short-Term Mortality of Congestive Heart Failure: A Retrospective Cohort Study[J]. Front Cardiovasc Med, 2021, 8: 753133. doi: 10.3389/fcvm.2021.753133
[20] Yamaguchi S, Abe M, Arakaki T, et al. Incremental Prognostic Value of Platelet Count in Patients With Acute Heart Failure-A Retrospective Observational Study[J]. Circ J, 2019, 83(3): 576-583.
[21] 中华医学会心血管病学分会, 中华心血管病杂志编辑委员会. 急性心力衰竭诊断和治疗指南[J]. 中华心血管病杂志, 2010, 38(3): 195-208. https://www.cnki.com.cn/Article/CJFDTOTAL-QKYL201005005.htm
[22] Matsushita K, Mahmoodi BK, Woodward M, et al. Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate[J]. JAMA, 2012, 307(18): 1941-1951.
[23] Breiman L. Random Forests[J]. Machine Learning, 2001, 45(1): 5-32.
[24] Vaduganathan M, Greene SJ, Butler J, et al. The immunological axis in heart failure: importance of the leukocyte differential[J]. Heart Fail Rev, 2013, 18(6): 835-845.
[25] Swirski FK, Nahrendorf M, Etzrodt M, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites[J]. Science, 2009, 325(5940): 612-616.
[26] Maisel AS, Knowlton KU, Fowler P, et al. Adrenergic control of circulating lymphocyte subpopulations. Effects of congestive heart failure, dynamic exercise, and terbutaline treatment[J]. J Clin Invest, 1990, 85(2): 462-467.
[27] Colotta F, Re F, Polentarutti N, et al. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products[J]. Blood, 1992, 80(8): 2012-2020.
[28] Raffaghello L, Bianchi G, Bertolotto M, et al. Human mesenchymal stem cells inhibit neutrophil apoptosis: a model for neutrophil preservation in the bone marrow niche[J]. Stem Cells, 2008, 26(1): 151-162.
[29] Tracchi I, Ghigliotti G, Mura M, et al. Increased neutrophil lifespan in patients with congestive heart failure[J]. Eur J Heart Fail, 2009, 11(4): 378-385.
[30] Silva N, Bettencourt P, GuimarãesJT. The lymphocyte-to-monocyte ratio: an added value for death prediction in heart failure[J]. Nutr Metab Cardiovasc Dis, 2015, 25(11): 1033-1040.
-




 下载:
下载: