Efficacy and safety of four SGLT2 inhibitors in the treatment of heart failure with reduced ejection fraction: a network meta-analysis
-
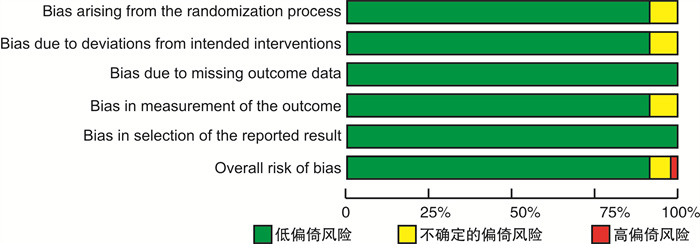
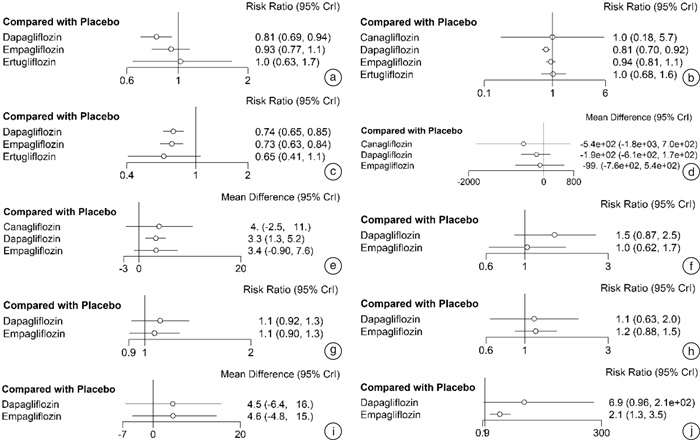
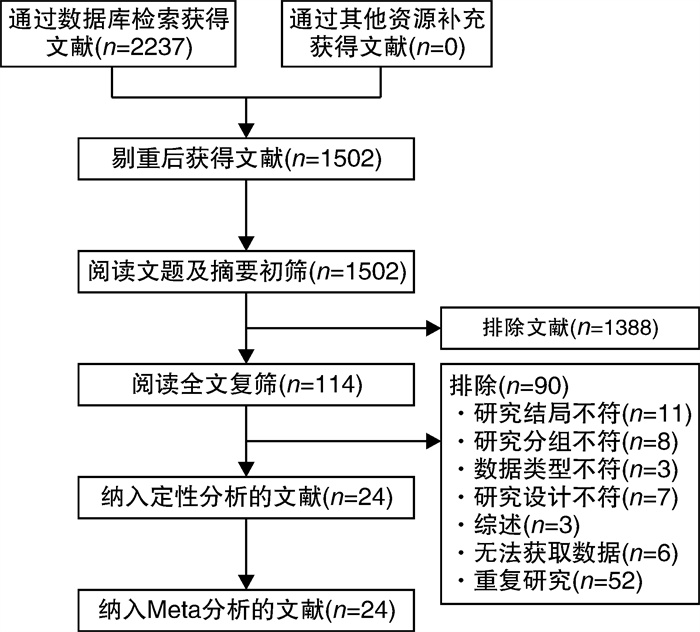
摘要: 目的 系统评价4种钠-葡萄糖协同转运蛋白2(SGLT2)抑制剂治疗射血分数降低的心力衰竭(HFrEF)患者的有效性和安全性。方法 计算机检索PubMed、EMBASE、The Cochrane Library、中国知网、维普和万方等数据库,搜集关于达格列净、卡格列净、恩格列净及艾托格列净4种SGLT2抑制剂治疗HFrEF患者的随机对照试验(RCT),检索时限均为建库至2022年7月1日。由2名研究者独立筛选文献、提取资料并评价纳入研究的偏倚风险后,采用R4.0.1软件进行网状meta分析。结果 共纳入24个RCT,包含11 972例HFrEF患者。网状meta分析结果显示:与安慰剂组比较,在降低心血管死亡和全因死亡发生率方面,达格列净更有优势(心血管死亡:RR=0.81,95%CI:0.69~0.94;全因死亡:RR=0.81,95%CI:0.70~0.92);在降低再入院发生率方面,达格列净和恩格列净更有优势(达格列净:RR=0.74,95%CI:0.65~0.85;恩格列净:RR=0.73,95%CI:0.63~0.84);在降低N末端B型利钠肽前体(NT-proBNP)方面,卡格列净、达格列净和恩格列净之间差异无统计学意义;在改善左心室射血分数(LVEF)方面,达格列净更有优势(MD=3.3,95%CI:1.27~5.29);在生殖器感染发生率方面,恩格列净发生风险更高(RR=2.09,95%CI:1.29~3.52);在低血糖、容量不足、尿路感染发生率及堪萨斯城心肌病问卷(KCCQ)评分方面,达格列净和恩格列净之间差异无统计学意义。累积排序概率图下面积(SUCRA)显示:在降低心血管死亡和全因死亡发生率方面,达格列净疗效排在第1位;在降低再入院发生率方面,艾托格列净疗效排在第1位;在降低NT-proBNP和改善LVEF方面,卡格列净疗效排在第1位;在提升KCCQ评分方面,恩格列净排疗效在第1位;在低血糖、容量不足、尿路感染、生殖器感染等不良反应发生率方面,不同SGLT2抑制剂安全性均劣于安慰剂。结论 当前证据表明,4种SGLT2抑制剂治疗HFrEF患者的有效性和安全性差异无统计学意义。无论使用哪一种SGLT2抑制剂均可以改善HFrEF患者预后,减少不良结局事件发生率,但同时存在低血糖、容量不足、尿路感染、生殖器感染等不良反应的发生风险。
-
关键词:
- 钠-葡萄糖协同转运蛋白2抑制剂 /
- 心力衰竭,射血分数降低的 /
- 网状meta分析 /
- 随机对照试验
Abstract: Objective To systematically review the efficacy and safety of sodium-glucose cotransporter 2(SGLT2) inhibitors in patients with heart failure with reduced ejection fraction(HFrEF).Methods PubMed, EMBASE, The Cochrane Library, CNKI, VIP, and WanFang Data databases were electronically searched to collect randomized controlled trials(RCTs) on the treatment of HFrEF with four SGLT2 inhibitors: dapagliflozin, canagliflozin, empagliflozin and itogliflozin from inception to July 1, 2022. Two reviewers independently screened literature, extracted data and assessed risk of bias of included studies. Then, network meta-analysis was performed by using R4.0. 1 software.Results A total of 24 RCTs involving 11 972 patients with HFrEF were included. The results of the network meta-analysis show that: compared with the placebo group, dapagliflozin was superior in reducing the incidence of cardiovascular mortality and all-cause mortality(cardiovascular mortality: RR=0.81, 95%CI: 0.69-0.94; all-cause mortality: RR=0.81, 95%CI: 0.70-0.92), dapagliflozin and empagliflozin superior in reducing the incidence of readmission(dapagliflozin: RR=0.74, 95%CI: 0.65-0.85; empagliflozin: RR=0.73, 95%CI: 0.63-0.84), there was no significant difference between canagliflozin, dapagliflozin, and empagliflozin in reducing the nerve terminal type B natriuretic peptide(NT-proBNP), dapagliflozin was superior in improving left ventricular ejection fraction(LVEF)(MD=3.3, 95%CI: 1.27-5.29), empagliflozin had a higher risk of genital infection(RR=2.09, 95%CI: 1.29-3.52), there was no significant difference between dapagliflozin and empagliflozin in the incidence of hypoglycemia, volume depletion, urinary tract infection and KCCQ score. The results of SUCRA show that: Dapagliflozin ranks first in terms of reducing the incidence of cardiovascular mortality and all-cause mortality; Ertugliflozin ranks first in terms of reducing the incidence of readmission; Canagliflozin ranks first in terms of reducing NT-proBNP and improving LVEF; Empagliflozin ranks first in terms of improving the KCCQ score; in terms of the incidence of hypoglycemia, volume depletion, urinary tract infection, and genital infection, the safety of different SGLT2 inhibitors was inferior to placebo.Conclusion Current evidence shows that there is no significant difference in the efficacy and safety among the four SGLT2 inhibitors in patients with HFrEF. Regardless of which SGLT2 inhibitor is used, the prognosis of patients with HFrEF can be improved and the incidence of adverse outcome events can be reduced, and also there are risks of adverse reactions such as hypoglycemia, volume depletion, urinary tract infection, and genital infection. -

-
表 1 纳入研究的基本特征
Table 1. Basic characteristics of the included studies
研究 HFrEF定义 NYHA分级 随访时间/月 样本量/例 试验组(样本量/例) 对照组(样本量/例) 结局指标 贾朋聪2021 LVEF < 40% Ⅱ~Ⅳ级 3 50 达格列净10mg+常规(26) 常规(24) ①③④
⑤⑥⑧魏云杰2020 LVEF < 40% Ⅱ~Ⅲ级 2 102 卡格列净100/300mg+常规(48) 常规(54) ①②④⑤ 郝正阳2021 LVEF < 40% \ 1.84 175 达格列净+二甲双胍(83) 二甲双胍(92) ①②③④
⑤⑥⑦汤曾耀2022 LVEF < 40% Ⅱ~Ⅲ级 1 96 达格列净5/10mg(48) 安慰剂(48) ⑤ 刘荣2022 LVEF < 40% Ⅱ~Ⅳ级 6 85 达格列净10mg(37) 常规(49) ③④⑤ 张虎2022 LVEF < 40% \ 1 83 达格列净10mg(41) 安慰剂(42) ④⑩ 李先芳2020 LVEF < 40% \ 2.75 102 达格列净5mg+常规(52) 常规(50) ④⑤ 郑黄生2021 LVEF≤40% Ⅱ~Ⅳ级 1 147 达格列净5/10mg+常规(73) 常规(74) ④⑤⑥⑦⑧ 杨攀2021 LVEF < 40% Ⅱ~Ⅳ级 6 104 达格列净10mg+常规(46) 常规(58) ③④⑤ 黄瑞娜2022 LVEF < 40% Ⅱ~Ⅳ级 2 62 达格列净10mg+常规(37) 常规(25) ④⑦⑧⑨ Santos-Gallego 2020 LVEF < 50% Ⅱ~Ⅲ级 6 84 恩格列净10mg(42) 安慰剂(42) ①②③⑤
⑥⑧⑨⑩Palau 2022 LVEF≤40% Ⅱ~Ⅲ/Ⅳ级 3 90 达格列净10mg(45) 安慰剂(45) ⑤ Packer 2020 LVEF≤40% Ⅱ~Ⅳ级 16 3730 恩格列净10mg(1863) 安慰剂(1867) ①②③④⑥
⑦⑧⑨⑩Reis 2022 LVEF < 50% Ⅱ~Ⅳ级 6 40 达格列净5mg+常规(20) 常规(20) ②④⑤
⑥⑦⑧Nassif 2019 LVEF≤40% Ⅱ~Ⅲ级 2.75 263 达格列净10mg(131) 安慰剂(132) ①②③④
⑥⑦⑩Jensen 2020 LVEF≤40% Ⅰ~Ⅲ级 2.75 190 恩格列净10mg(95) 安慰剂(95) ②③⑥
⑦⑧⑨Lee 2020 LVEF≤40% Ⅱ~Ⅳ级 8.26 105 恩格列净10mg(52) 安慰剂(53) ④⑤⑦
⑧⑨⑩Abraham 2021 LVEF≤40% Ⅱ~Ⅳ级 2.75 312 恩格列净10mg(156) 安慰剂(156) ②④⑥⑦
⑧⑨⑩McMurray 2019 LVEF≤40% Ⅱ~Ⅳ级 18.2 4744 达格列净10mg(2373) 安慰剂(2371) ①②③④⑥
⑦⑧⑨⑩Singh 2020 LVEF < 45% Ⅰ~Ⅲ级 12 56 达格列净10mg(28) 安慰剂(28) ①②③⑤ Mordi 2020 LVEF < 50% Ⅱ~Ⅳ级 1.38 23 恩格列净25mg(23) 安慰剂(23) ④ Kato 2019 EF < 45% Ⅰ~Ⅲ级 50.4 671 达格列净10mg(318) 安慰剂(353) ①②③⑥
⑦⑧⑨Spertus 2022 LVEF≤40% \ 2.75 180 卡格列净100mg(90) 安慰剂(90) ② Cosentino 2020 LVEF≤45% Ⅰ~Ⅲ级 42 478 艾托格列净5/15mg(319) 安慰剂(159) ①②③ 注:①:心血管死亡发生率;②:全因死亡发生率;③:再入院发生率;④:NT-proBNP;⑤:治疗后LVEF变化;⑥:低血糖;⑦:容量不足;⑧:尿路感染;⑨:生殖器感染;⑩:KCCQ评分。 表 2 HFrEF患者4种药物疗效的概率排名
Table 2. Ranking probability of the efficacy of four drug in patients with HFrEF
排名 心血管死亡 全因死亡 再入院 NT-proBNP 治疗后LVEF变化 低血糖 容量不足 尿路感染 生殖器感染 KCCQ评分 1 达格列净(74%) 达格列净(49%) 艾托格列净(62%) 卡格列净(65%) 卡格列净(48%) 安慰剂(51%) 安慰剂(66%) 安慰剂(56%) 安慰剂(97%) 恩格列净(69%) 2 恩格列净(46%) 恩格列净(28%) 恩格列净(46%) 达格列净(46%) 达格列净(46%) 恩格列净(41%) 恩格列净(42%) 恩格列净(40%) 恩格列净(86%) 达格列净(67%) 3 安慰剂(52%) 安慰剂(32%) 达格列净(42%) 安慰剂(43%) 恩格列净(31%) 达格列净(81%) 达格列净(60%) 达格列净(47%) 达格列净(86%) 安慰剂(14%) 4 艾托格列净(52%) 艾托格列净(27%) 安慰剂(96%) 恩格列净(33%) 安慰剂(85%) 5 卡格列净(45%) -
[1] Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines[J]. Circulation, 2022, 145(18): e895-e1032.
[2] 王华, 梁延春. 中国心力衰竭诊断和治疗指南2018[J]. 中华心血管病杂志, 2018, 46(10): 760-789. https://www.cnki.com.cn/Article/CJFDTOTAL-ZLYS201904009.htm
[3] Roger VL. Epidemiology of heart failure: A contemporary perspective[J]. Circ Res, 2021, 128(10): 1421-1434. doi: 10.1161/CIRCRESAHA.121.318172
[4] Zhang Y, Zhang J, Butler J, et al. Contemporary epidemiology, management, and outcomes of patients hospitalized for heart failure in China: results from the China heart failure(China-HF)registry[J]. J Card Fail, 2017, 23(12): 868-875. doi: 10.1016/j.cardfail.2017.09.014
[5] Lahnwong S, Palee S, Apaijai N, et al. Acute dapagliflozin administration exerts cardioprotective effects in rats with cardiac ischemia/reperfusion injury[J]. Cardiovasc Diabetol, 2020, 19(1): 91. doi: 10.1186/s12933-020-01066-9
[6] McMurray J, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction[J]. N Engl J Med, 2019, 381(21): 1995-2008. doi: 10.1056/NEJMoa1911303
[7] Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure[J]. N Engl J Med, 2020, 383(15): 1413-1424. doi: 10.1056/NEJMoa2022190
[8] Zannad F, Ferreira JP, Pocock SJ, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials[J]. Lancet, 2020, 396(10254): 819-829. doi: 10.1016/S0140-6736(20)31824-9
[9] 中国心力衰竭中心联盟专家委员会. 心力衰竭SGLT2抑制剂临床应用的中国专家共识[J]. 临床心血管病杂志, 2022, 38(8): 599-605. https://lcxxg.whuhzzs.com/article/doi/10.13201/j.issn.1001-1439.2022.08.001
[10] O'Meara E, McDonald M, Chan M, et al. CCS/CHFS Heart Failure Guidelines: Clinical Trial Update on Functional Mitral Regurgitation, SGLT2 Inhibitors, ARNI in HFpEF, and Tafamidis in Amyloidosis[J]. Can J Cardiol, 2020, 36(2): 159-169. doi: 10.1016/j.cjca.2019.11.036
[11] 葛均波, 霍勇, 高秀芳, 等. 改善心血管和肾脏结局的新型抗高血糖药物临床应用中国专家建议[J]. 中国循环杂志, 2020, 35(3): 231-238. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGXH202003002.htm
[12] Sterne J, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials[J]. BMJ, 2019, 366: l4898.
[13] 刘津池, 刘畅, 华成舸. 随机对照试验偏倚风险评价工具RoB2(2019修订版)解读[J]. 中国循证医学杂志, 2021, 21(6): 737-744. https://www.cnki.com.cn/Article/CJFDTOTAL-ZZXZ202106017.htm
[14] 葛龙. 可切除胃癌辅助化疗及其应答敏感基因筛选的研究[D]. 兰州大学, 2019.
[15] 陈卉卉. 三阴性乳腺癌预后预测模型的建立及不同靶向治疗方案的网络meta分析[D]. 浙江大学, 2020.
[16] 贾朋聪. 达格列净对老年射血分数降低的心力衰竭合并2型糖尿病患者的临床研究[D]. 河北医科大学, 2021.
[17] 魏云杰, 王俊峰, 程飞, 等. 卡格列净治疗女性糖尿病伴射血分数降低的心力衰竭患者的临床疗效及其作用机制研究[J]. 实用心脑肺血管病杂志, 2020, 28(9): 26-29+39. https://www.cnki.com.cn/Article/CJFDTOTAL-SYXL202009008.htm
[18] 郝正阳, 张彦周. 达格列净对慢性射血分数降低性心力衰竭合并2型糖尿病患者心功能的影响[J]. 河南医学研究, 2021, 30(32): 5990-5994. https://www.cnki.com.cn/Article/CJFDTOTAL-HNYX202132010.htm
[19] 汤曾耀. 达格列净在射血分数降低的心力衰竭患者中的临床观察[J]. 当代医学, 2022, 28(11): 86-88. https://www.cnki.com.cn/Article/CJFDTOTAL-DDYI202211027.htm
[20] 刘荣, 张鑫, 于海波, 等. 达格列净对射血分数降低心力衰竭并植入心脏除颤起搏器患者心功能及室性心律失常影响[J]. 临床军医杂志, 2022, 50(5): 445-448. https://www.cnki.com.cn/Article/CJFDTOTAL-JYGZ202205002.htm
[21] 张虎, 谭伟, 阮佩, 等. 达格列净片联合麝香保心丸治疗射血分数降低心力衰竭急性发作期的临床疗效观察[J]. 中国实验方剂学杂志, 2022, 28(17): 98-105. https://www.cnki.com.cn/Article/CJFDTOTAL-ZSFX202217013.htm
[22] 李先芳, 林璋. 达格列净在老年射血分数下降性心力衰竭合并2型糖尿病患者中的临床价值[J]. 中国医药指南, 2020, 18(35): 1-3. https://www.cnki.com.cn/Article/CJFDTOTAL-YYXK202035002.htm
[23] 郑黄生, 周炳凤. 钠-葡萄糖协同转运蛋白-2抑制剂治疗射血分数降低心力衰竭的疗效及预后分析[J]. 医学信息, 2021, 34(10): 92-96. https://www.cnki.com.cn/Article/CJFDTOTAL-YXXX202110026.htm
[24] 杨攀, 张琼, 王学影. 达格列净治疗射血分数降低心衰患者的疗效观察[J]. 广西医科大学学报, 2021, 38(7): 1436-1441. https://www.cnki.com.cn/Article/CJFDTOTAL-GXYD202107030.htm
[25] 黄瑞娜, 李日健, 黄春燕, 等. 短期使用达格列净对射血分数下降型心力衰竭合并2型糖尿病患者临床疗效的影响[J]. 中国药物经济学, 2022, 17(2): 98-101. https://www.cnki.com.cn/Article/CJFDTOTAL-ZYWA202202025.htm
[26] Santos-Gallego CG, Vargas-Delgado AP, Requena-Ibanez JA, et al. Randomized trial of empagliflozin in nondiabetic patients with heart failure and reduced ejection fraction[J]. J Am Coll Cardiol, 2021, 77(3): 243-255.
[27] Palau P, Amiguet M, Domínguez E, et al. Short-term effects of dapagliflozin on maximal functional capacity in heart failure with reduced ejection fraction(DAPA-VO2): a randomized clinical trial[J]. Eur J Heart Fail, 2022, 24(10): 1816-1826.
[28] Reis J, Teixeira AR, Gonçalves AV, et al. Dapagliflozin impact on the exercise capacity of non-diabetic heart failure with reduced ejection fraction patients[J]. J Clin Med, 2022, 11(10).
[29] Nassif ME, Windsor SL, Tang F, et al. Dapagliflozin effects on biomarkers, symptoms, and functional status in patients with heart failure with reduced ejection fraction: The DEFINE-HF Trial[J]. Circulation, 2019, 140(18): 1463-1476.
[30] Jensen J, Omar M, Kistorp C, et al. Twelve weeks of treatment with empagliflozin in patients with heart failure and reduced ejection fraction: A double-blinded, randomized, and placebo-controlled trial[J]. Am Heart J, 2020, 228: 47-56.
[31] Lee M, Brooksbank K, Wetherall K, et al. Effect of empagliflozin on left ventricular volumes in patients with type 2 diabetes, or prediabetes, and heart failure with reduced ejection fraction(SUGAR-DM-HF)[J]. Circulation, 2021, 143(6): 516-525.
[32] Abraham WT, Lindenfeld J, Ponikowski P, et al. Effect of empagliflozin on exercise ability and symptoms in heart failure patients with reduced and preserved ejection fraction, with and without type 2 diabetes[J]. Eur Heart J, 2021, 42(6): 700-710.
[33] Singh J, Mordi IR, Vickneson K, et al. Dapagliflozin versus placebo on left ventricular remodeling in patients with diabetes and heart failure: The REFORM Trial[J]. Diabetes Care, 2020, 43(6): 1356-1359.
[34] Mordi NA, Mordi IR, Singh JS, et al. Renal and cardiovascular effects of SGLT2 inhibition in combination with loop diuretics in patients with type 2 diabetes and chronic heart failure: The RECEDE-CHF Trial[J]. Circulation, 2020, 142(18): 1713-1724.
[35] Kato ET, Silverman MG, Mosenzon O, et al. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus[J]. Circulation, 2019, 139(22): 2528-2536.
[36] Spertus JA, Birmingham MC, Nassif M, et al. The SGLT2 inhibitor canagliflozin in heart failure: the CHIEF-HF remote, patient-centered randomized trial[J]. Nat Med, 2022, 28(4): 809-813.
[37] Cosentino F, Cannon CP, Cherney D, et al. Efficacy of ertugliflozin on heart failure-related events in patients with type 2 diabetes mellitus and established atherosclerotic cardiovascular disease: results of the VERTIS CV Trial[J]. Circulation, 2020, 142(23): 2205-2215.
[38] 廖梦阳, 廖玉华, 余淼, 等. SGLT2抑制剂治疗心力衰竭潜在机制的新认识[J]. 临床心血管病杂志, 2022, 38(1): 1-6. https://lcxxg.whuhzzs.com/article/doi/10.13201/j.issn.1001-1439.2022.01.001
[39] Home PD, Pocock SJ, Beck-Nielsen H, et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes(RECORD): a multicentre, randomised, open-label trial[J]. Lancet, 2009, 373(9681): 2125-2135.
[40] Maruyama T, Takashima H, Oguma H, et al. Canagliflozin improves erythropoiesis in diabetes patients with anemia of chronic kidney disease[J]. Diabetes Technol Ther, 2019, 21(12): 713-720.
[41] Tang J, Ye L, Yan Q, et al. Effects of sodium-glucose cotransporter 2 inhibitors on water and sodium metabolism[J]. Front Pharmacol, 2022, 13: 800490.
[42] Curtain JP, Docherty KF, Jhund PS, et al. Effect of dapagliflozin on ventricular arrhythmias, resuscitated cardiac arrest, or sudden death in DAPA-HF[J]. Eur Heart J, 2021, 42(36): 3727-3738.
[43] Zhou H, Wang S, Zhu P, et al. Empagliflozin rescues diabetic myocardial microvascular injury via AMPK-mediated inhibition of mitochondrial fission[J]. Redox Biol, 2018, 15: 335-346.
[44] Scheen AJ. An update on the safety of SGLT2 inhibitors[J]. Expert Opin Drug Saf, 2019, 18(4): 295-311.
[45] Dave CV, Schneeweiss S, Patorno E. Comparative risk of genital infections associated with sodium-glucose co-transporter-2 inhibitors[J]. Diabetes Obes Metab, 2019, 21(2): 434-438.
-




 下载:
下载: