Correlation analysis between p.R616C mutation of JPH2 gene and familial dilated cardiomyopathy
-
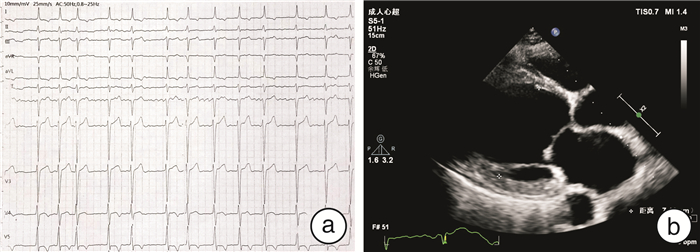
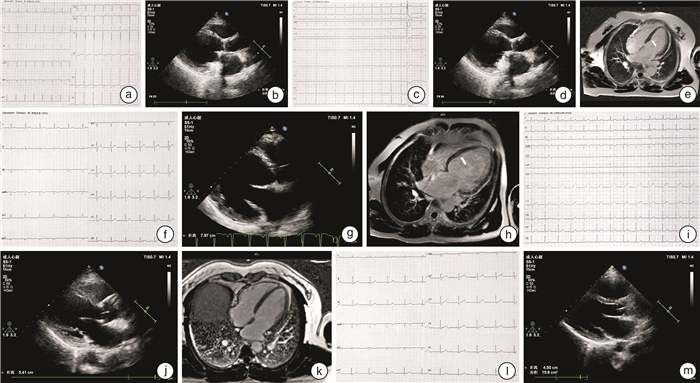
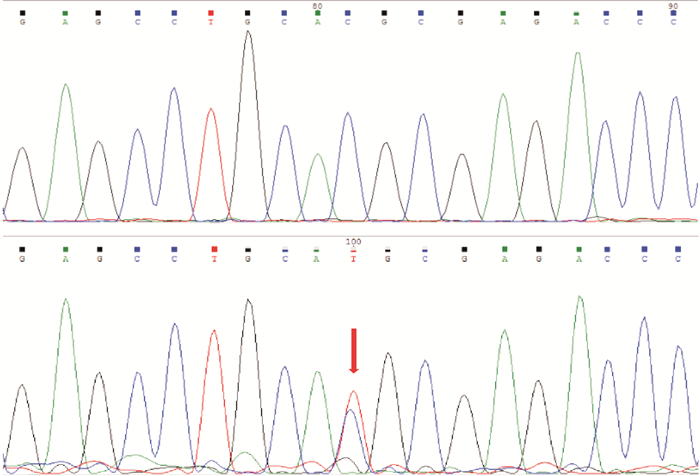
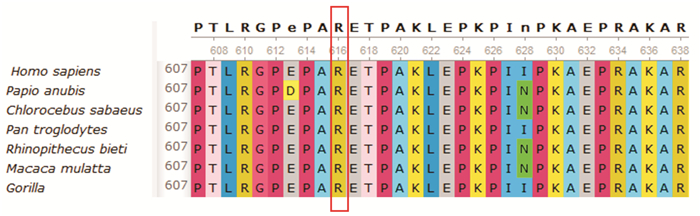
摘要: 目的 对1例家族性扩张型心肌病(dilated cardiomyopathy,DCM)家系进行致病基因筛查,分析其基因型与表型的相关性。方法 研究对象为1例DCM先证者及其家系成员,另选取体检中心健康成年人为对照。收集先证者家系的临床资料,对先证者及发病家系成员进行全基因组测序,筛选可疑致病基因,并通过Sanger测序法进行验证。对其他家系成员及对照组进行相关基因筛查。结果 对该家系筛查发现包括先证者在内的5例DCM患者,且基因检测发现均存在JPH2基因的c.1846C>T(p.Arg616Cys)突变,另有1例无临床表型的突变基因携带者。余家系成员及对照组相同位点未见异常。在随访过程中先证者出现恶性心律失常。结论 本研究发现JPH2基因的p.R616C突变可能导致家族性DCM,且该基因突变可能为恶性基因突变。Abstract: Objective The aim was to screen the pathogenic genes and analyze the genotype-phenotype correlation in a pedigree with dilated cardiomyopathy.Methods The proband with dilated cardiomyopathy(DCM)and his family members were included in this study, while healthy controls from physical examination centers were selected. Clinical data were collected through genetic counseling. The whole genome testing was carried out to screen for suspected pathogenic genes in the proband and affected members. Candidate mutations were further confirmed by Sanger sequencing. Genetic testing was performed on other family members and controls.Results Five patients with DCM were identified in this family, and the same mutation in the JPH2 gene(c.1846C>T/p.Arg616Cys) was found in all of them. There was also one carrier with no obvious clinical phenotype. This variant was absent in other family members and controls. The proband developed malignant arrhythmia during follow-up.Conclusion In this study, we find that JPH2 p.R616C variant probably contributes to familial dilated cardiomyopathy, and the mutation may be malignant.
-
Key words:
- dilated cardiomyopathy /
- JPH2 /
- gene mutation
-

-
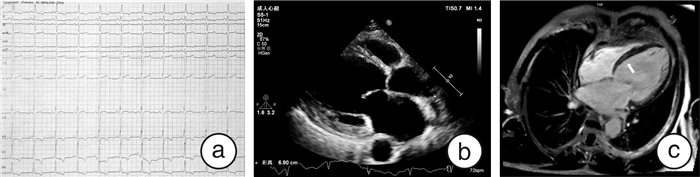
表 1 先证者家系临床资料
Table 1. Clinical data of the proband's family
成员 性别 年龄/岁 症状 ECG UCG CMR纤维化 LVEDd/mm LVEF/% Ⅱ-1 男 67 活动后胸闷气喘 房颤 71 13 + Ⅱ-5 女 61 活动后胸闷气喘 窦性心律;ST-T改变 59 34 未做 Ⅱ-9 女 55 心慌伴胸闷气喘 窦性心律;室性期前收缩 57 40 + Ⅲ-1 男 43 活动后胸闷气喘 窦性心律 80 19 + Ⅲ-3 女 41 - 窦性心律 54 43 - Ⅳ-3 女 19 - 窦性心律 45 64 未做 -
[1] Mckenna WJ, Judge DP. Epidemiology of the inherited cardiomyopathies[J]. Nat Rev Cardiol, 2021, 18(1): 22-36. doi: 10.1038/s41569-020-0428-2
[2] Schulz LP, Vischer AS. Cardiomyopathies in the Clinical Practice-an Overview[J]. Praxis(Bern 1994), 2022, 111(11): 623-631. doi: 10.1024/1661-8157/a003912
[3] Huang YS, Xing YL, Li HW. Heterozygous desmin gene(DES)mutation contributes to familial dilated cardiomyopathy[J]. J Int Med Res, 2021, 49(4): 3000605211006598.
[4] Sabater-Molina M, Navarro M, Garcia-Molina Saez E, et al. Mutation in JPH2 cause dilated cardiomyopathy[J]. Clin Genet, 2016, 90(5): 468-469. doi: 10.1111/cge.12825
[5] Jones EG, Mazaheri N, Maroofian R, et al. Analysis of enriched rare variants in JPH2-encoded junctophilin-2 among Greater Middle Eastern individuals reveals a novel homozygous variant associated with neonatal dilated cardiomyopathy[J]. Sci Rep, 2019, 9(1): 9038. doi: 10.1038/s41598-019-44987-6
[6] Miura A, Kondo H, Yamamoto T, et al. Sudden Unexpected Death of Infantile Dilated Cardiomyopathy with JPH2 and PKD1 Gene Variants[J]. Int Heart J, 2020, 61(5): 1079-1083. doi: 10.1536/ihj.20-155
[7] Walsh R, Offerhaus JA, Tadros R, et al. Minor hypertrophic cardiomyopathy genes, major insights into the genetics of cardiomyopathies[J]. Nat Rev Cardiol, 2022, 19(3): 151-167. doi: 10.1038/s41569-021-00608-2
[8] Patel V, Chahal C. Editorial commentary: Genomic and precision medicine provides deeper insights into the genetic basis of diverse JPH2-mediated phenotypes[J]. Trends Cardiovasc Med, 2023, 33(1): 11-12. doi: 10.1016/j.tcm.2021.12.010
[9] Jordan E, Peterson L, Ai T, et al. Evidence-Based Assessment of Genes in Dilated Cardiomyopathy[J]. Circulation, 2021, 144(1): 7-19. doi: 10.1161/CIRCULATIONAHA.120.053033
[10] 中华医学会心血管病学分会, 中国心肌炎心肌病协作组. 中国扩张型心肌病诊断和治疗指南[J]. 临床心血管病杂志, 2018, 34(5): 421-434. doi: 10.13201/j.issn.1001-1439.2018.05.002
[11] Njoroge JN, Mangena JC, Aribeana C, et al. Emerging Genotype-Phenotype Associations in Dilated Cardiomyopathy[J]. Curr Cardiol Rep, 2022, 24(9): 1077-1084. doi: 10.1007/s11886-022-01727-z
[12] Schultheiss HP, Fairweather D, Caforio ALP, et al. Dilated cardiomyopathy[J]. Nat Rev Dis Primers, 2019, 5(1): 32. doi: 10.1038/s41572-019-0084-1
[13] Peters S, Johnson R, Birch S, et al. Familial Dilated Cardiomyopathy[J]. Heart Lung Circ, 2020, 29(4): 566-574. doi: 10.1016/j.hlc.2019.11.018
[14] 陈香璇, 程维礼, 张郁青, 等. TTN及MYH7多基因突变与一家族性扩张型心肌病的相关性分析[J]. 临床心血管病杂志, 2021, 37(8): 744-748. doi: 10.13201/j.issn.1001-1439.2021.08.014
[15] Chen SN, Mestroni L, Taylor MRG. Genetics of dilated cardiomyopathy[J]. Curr Opin Cardiol, 2021, 36(3): 288-294. doi: 10.1097/HCO.0000000000000845
[16] Mazzarotto F, Tayal U, Buchan RJ, et al. Reevaluating the Genetic Contribution of Monogenic Dilated Cardiomyopathy[J]. Circulation, 2020, 141(5): 387-398. doi: 10.1161/CIRCULATIONAHA.119.037661
[17] Lehnart SE, Wehrens XHT. The role of junctophilin proteins in cellular function[J]. Physiol Rev, 2022, 102(3): 1211-1261. doi: 10.1152/physrev.00024.2021
[18] Setterberg IE, Le C, Frisk M, et al. The Physiology and Pathophysiology of T-Tubules in the Heart[J]. Front Physiol, 2021, 12: 718404. doi: 10.3389/fphys.2021.718404
[19] Douard M, Brette F. Transverse tubules strike back: may the junctophilin-2 be with you[J]. Cardiovasc Res, 2021, 117(1): 7-8. doi: 10.1093/cvr/cvaa115
[20] Poulet C, Sanchez-Alonso J, Swiatlowska P, et al. Junctophilin-2 tethers T-tubules and recruits functional L-type calcium channels to lipid rafts in adult cardiomyocytes[J]. Cardiovasc Res, 2021, 117(1): 149-161. doi: 10.1093/cvr/cvaa033
[21] Yamakawa S, Wu D, Dasgupta M, et al. Role of t-tubule remodeling on mechanisms of abnormal calcium release during heart failure development in canine ventricle[J]. Am J Physiol Heart Circ Physiol, 2021, 320(4): H1658-H1669. doi: 10.1152/ajpheart.00946.2020
[22] Parker LE, Kramer RJ, Kaplan S, et al. One gene, two modes of inheritance, four diseases: A systematic review of the cardiac manifestation of pathogenic variants in JPH2-encoded junctophilin-2[J]. Trends Cardiovasc Med, 2023, 33(1): 1-10. doi: 10.1016/j.tcm.2021.11.006
[23] Luo T, Yan N, Xu M, et al. Junctophilin-2 allosterically interacts with ryanodine receptor type 2 to regulate calcium release units in mouse cardiomyocytes[J]. Gen Physiol Biophys, 2022, 41(2): 133-140.
[24] Dibb KM, Louch WE, Trafford AW. Cardiac Transverse Tubules in Physiology and Heart Failure[J]. Annu Rev Physiol, 2022, 84: 229-255. doi: 10.1146/annurev-physiol-061121-040148
-




 下载:
下载: