-
摘要: 免疫检查点抑制剂(immune checkpoint inhibitor,ICI)是肿瘤治疗的一大突破,在临床应用日益广泛。ICI相关心肌炎是ICI药物的罕见不良反应之一,病死率较高。尽管国内外均报道了该不良反应的治疗经验和指南,但部分医生在临床工作中仍存在认知不足、监管评估方案缺乏等问题。故本文对ICI相关心肌炎发病机制、诊断、治疗策略进行综述,以提高肿瘤科及心脏科医师的认识。Abstract: Immune checkpoint inhibitors (ICI) are a major breakthrough in oncology treatment and are increasingly used in clinical practice. ICI-associated myocarditis is one of the rare adverse reactions to ICI drugs, with a high mortality rate. Guidelines for treating this adverse reaction have been written both domestically and internationally at home and abroad. However, there is still a lack of awareness and reliable regulatory assessment protocols in clinical work. Therefore, this article reviews the pathogenesis, diagnosis, and treatment strategies of ICI-associated myocarditis in order to raise the awareness of oncologists and cardiologists.
-
Key words:
- myocarditis /
- immune checkpoint inhibitor
-

-
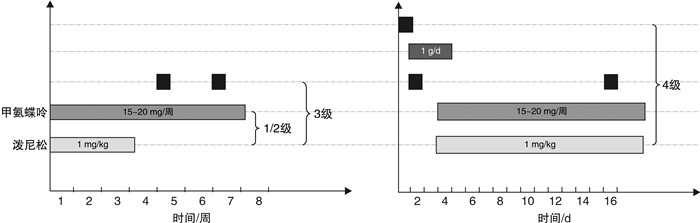
图 1 ICI相关心肌炎治疗新模式[40]
Figure 1. New model of treatment for ICI-associated myocarditis
表 1 ICI相关心肌炎分级及治疗
Table 1. Grading and treatment of ICI-associated myocarditis
项目 1级 2级 3级 4级 症状 亚临床心肌损伤 轻微症状 明显的心血管症状 明显的心血管症状或危及生命 辅助检查 心脏损伤标志物轻度升高,心电图异常 心肌标志物水平升高,心电图异常 心脏彩超提示LVEF<50%或室壁局部节段运动异常,CRB确诊或疑似诊断为心肌炎 如心电图提示室性心动过速,心脏彩超提示严重的心功能下降等危及生命的检查 重启免疫 病情稳定后可重启免疫治疗 病情稳定后可重启免疫治疗 有3级心肌炎、肺炎、肾炎、肝炎和严重神经系统毒性者永久停用ICI;若患者心肌炎病情稳定后评估后续免疫治疗获益较大,则可重启免疫治疗 永不重启免疫治疗 多学科会诊 心血管科会诊 心血管科会诊,积极处置基础疾病 多学科会诊 多学科会诊 治疗 甲泼尼龙1~4 mg/kg/d持续3~5 d,后逐渐减量,心脏损伤指标恢复基线水平后继续激素治疗2~4周 甲泼尼龙1~4 mg/kg/d持续3~5 d,后逐渐减量,心脏损伤指标恢复基线水平后继续激素治疗2~4周 甲泼尼龙500~1 000 mg/d,持续3~5 d,后逐渐减量,心脏损伤指标恢复基线水平后继续激素治疗4周 大剂量激素脉冲治疗24 h无效考虑加用ATG/英夫利西单抗(重度心力衰竭患者禁用) -
[1] Robert C. A decade of immune-checkpoint inhibitors in cancer therapy[J]. Nat Commun, 2020, 11(1): 3801. doi: 10.1038/s41467-020-17670-y
[2] de Miguel M, Calvo E. Clinical Challenges of Immune Checkpoint Inhibitors[J]. Cancer Cell, 2020, 38(3): 326-333. doi: 10.1016/j.ccell.2020.07.004
[3] Tazdait M, Mezquita L, Lahmar J, et al. Patterns of responses in metastatic NSCLC during PD-1 or PDL-1 inhibitor therapy: Comparison of RECIST 1.1, irRECIST and iRECIST criteria[J]. Eur J Cancer, 2018, 88: 38-47. doi: 10.1016/j.ejca.2017.10.017
[4] Salem JE, Manouchehri A, Moey M, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study[J]. Lancet Oncol, 2018, 19(12): 1579-1589. doi: 10.1016/S1470-2045(18)30608-9
[5] Dimmitt SB, Stampfer HG, Martin JH, et al. Clinical benefits of evolocumab appear less than hoped[J]. Lancet, 2018, 391(10124): 933-934.
[6] Mahmood SS, Fradley MG, Cohen JV, et al. Myocarditis in Patients Treated With Immune Checkpoint Inhibitors[J]. J Am Coll Cardiol, 2018, 71(16): 1755-1764. doi: 10.1016/j.jacc.2018.02.037
[7] Moslehi JJ, Salem JE, Sosman JA, et al. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis[J]. Lancet, 2018, 391(10124): 933.
[8] Escudier M, Cautela J, Malissen N, et al. Clinical Features, Management, and Outcomes of Immune Checkpoint Inhibitor-Related Cardiotoxicity[J]. Circulation, 2017, 136(21): 2085-2087. doi: 10.1161/CIRCULATIONAHA.117.030571
[9] 陈炳秀, 吴代琴, 吴立荣, 等. 咽喉癌化疗后免疫检查点抑制剂相关心肌炎1例[J]. 临床心血管病杂志, 2023, 39(1): 76-80. doi: 10.13201/j.issn.1001-1439.2023.01.014
[10] Atkins MB, Lee SJ, Chmielowski B, et al. Combination Dabrafenib and Trametinib Versus Combination Nivolumab and Ipilimumab for Patients With Advanced BRAF-Mutant Melanoma: The DREAMseq Trial-ECOG-ACRIN EA6134[J]. J Clin Oncol, 2023, 41(2): 186-197. doi: 10.1200/JCO.22.01763
[11] Khalid AB, Calderon G, Jalal SI, et al. Physician Awareness of Immune-Related Adverse Events of Immune Checkpoint Inhibitors[J]. J Natl Compr Canc Netw, 2022, 20(12): 1316-1320.
[12] Stein-Merlob AF, Rothberg MV, Holman P, et al. Immunotherapy-Associated Cardiotoxicity of Immune Checkpoint Inhibitors and Chimeric Antigen Receptor T Cell Therapy: Diagnostic and Management Challenges and Strategies[J]. Curr Cardiol Rep, 2021, 23(3): 11. doi: 10.1007/s11886-021-01440-3
[13] Varricchi G, Galdiero MR, Marone G, et al. Cardiotoxicity of immune checkpoint inhibitors[J]. ESMO Open, 2017, 2(4): e000247. doi: 10.1136/esmoopen-2017-000247
[14] Johnson DB, Balko JM, Compton ML, et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade[J]. N Engl J Med, 2016, 375(18): 1749-1755. doi: 10.1056/NEJMoa1609214
[15] Green CE, Chacon J, Godinich BM, et al. The Heart of the Matter: Immune Checkpoint Inhibitors and Immune-Related Adverse Events on the Cardiovascular System[J]. Cancers(Basel), 2023, 15(24): 5707.
[16] 刘德敏, 路旭阳, 谷国强. 免疫检查点抑制剂致心脏毒性的研究进展[J]. 临床心血管病杂志, 2023, 39(6): 481-486. doi: 10.13201/j.issn.1001-1439.2023.06.014
[17] Khunger A, Battel L, Wadhawan A, et al. New Insights into Mechanisms of Immune Checkpoint Inhibitor-Induced Cardiovascular Toxicity[J]. Curr Oncol Rep, 2020, 22(7): 65. doi: 10.1007/s11912-020-00925-8
[18] Li H, Sun X, Sun D, et al. Thromboembolic events associated with immune checkpoint inhibitors: A real-world study of data from the food and drug administration adverse event reporting system(FAERS)database[J]. Int Immunopharmacol, 2021, 98: 107818. doi: 10.1016/j.intimp.2021.107818
[19] Mir H, Alhussein M, Alrashidi S, et al. Cardiac Complications Associated With Checkpoint Inhibition: A Systematic Review of the Literature in an Important Emerging Area[J]. Can J Cardiol, 2018, 34(8): 1059-1068. doi: 10.1016/j.cjca.2018.03.012
[20] Zhang L, Awadalla M, Mahmood SS, et al. Cardiovascular magnetic resonance in immune checkpoint inhibitor-associated myocarditis[J]. Eur Heart J, 2020, 41(18): 1733-1743. doi: 10.1093/eurheartj/ehaa051
[21] Ganatra S, Neilan TG. Immune Checkpoint Inhibitor-Associated Myocarditis[J]. Oncologist, 2018, 23(8): 879-886. doi: 10.1634/theoncologist.2018-0130
[22] Blanco-Domínguez R, Sánchez-Díaz R, de la Fuente H, et al. A Novel Circulating MicroRNA for the Detection of Acute Myocarditis[J]. N Engl J Med, 2021, 384(21): 2014-2027. doi: 10.1056/NEJMoa2003608
[23] Haanen J, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up[J]. Ann Oncol, 2018, 29(Suppl 4): ⅳ264-ⅳ266.
[24] Brahmer JR, Lacchetti C, Thompson JA. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline Summary[J]. J Oncol Pract, 2018, 14(4): 247-249. doi: 10.1200/JOP.18.00005
[25] Thompson JA, Schneider BJ, Brahmer J, et al. Management of Immunotherapy-Related Toxicities, Version 1.2022, NCCN Clinical Practice Guidelines in Oncology[J]. J Natl Compr Canc Netw, 2022, 20(4): 387-405. doi: 10.6004/jnccn.2022.0020
[26] Thompson JA, Schneider BJ, Brahmer J, et al. NCCN Guidelines Insights: Management of Immunotherapy-Related Toxicities, Version 1.2020[J]. J Natl Compr Canc Netw, 2020, 18(3): 230-241. doi: 10.6004/jnccn.2020.0012
[27] Haanen J, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up[J]. Ann Oncol, 2017, 28(suppl_4): ⅳ119-ⅳ142.
[28] Chalabi M, Cardona A, Nagarkar DR, et al. Efficacy of chemotherapy and atezolizumab in patients with non-small-cell lung cancer receiving antibiotics and proton pump inhibitors: pooled post hoc analyses of the OAK and POPLAR trials[J]. Ann Oncol, 2020, 31(4): 525-531. doi: 10.1016/j.annonc.2020.01.006
[29] Shirakawa K, Sano M. Osteopontin in Cardiovascular Diseases[J]. Biomolecules, 2021, 11(7): 1047.
[30] Jespersen MS, Fanø S, Stenør C, et al. A case report of immune checkpoint inhibitor-related steroid-refractory myocarditis and myasthenia gravis-like myositis treated with abatacept and mycophenolate mofetil[J]. Eur Heart J Case Rep, 2021, 5(11): ytab342. doi: 10.1093/ehjcr/ytab342
[31] Okuda K, Fu HY, Matsuzaki T, et al. Targeted Therapy for Acute Autoimmune Myocarditis with Nano-Sized Liposomal FK506 in Rats[J]. PLoS One, 2016, 11(8): e0160944.
[32] Zhang RS, Padegimas A, Murphy KM, et al. Treatment of corticosteroid refractory immune checkpoint inhibitor myocarditis with Infliximab: a case series[J]. Cardiooncology, 2021, 7(1): 13.
[33] Padegimas A, Agarwal P, Fleitman J, et al. Case Series of Ventricular Tachycardia and Myocarditis From Programmed Cell-Death Protein-1 Inhibitor Treated With Infliximab[J]. JACC Clin Electrophysiol, 2019, 5(8): 989-992.
[34] Esfahani K, Buhlaiga N, Thébault P, et al. Alemtuzumab for Immune-Related Myocarditis Due to PD-1 Therapy[J]. N Engl J Med, 2019, 380(24): 2375-2376.
[35] Salem JE, Allenbach Y, Vozy A, et al. Abatacept for Severe Immune Checkpoint Inhibitor-Associated Myocarditis[J]. N Engl J Med, 2019, 380(24): 2377-2379.
[36] Nguyen LS, Bretagne M, Arrondeau J, et al. Reversal of immune-checkpoint inhibitor fulminant myocarditis using personalized-dose-adjusted abatacept and ruxolitinib: proof of concept[J]. J Immunother Cancer, 2022, 10(4): e004699.
[37] Dolladille C, Akroun J, Morice PM, et al. Cardiovascular immunotoxicities associated with immune checkpoint inhibitors: a safety meta-analysis[J]. Eur Heart J, 2021, 42(48): 4964-4977.
[38] Schiopu S, Käsmann L, Schönermarck U, et al. Pembrolizumab-induced myocarditis in a patient with malignant mesothelioma: plasma exchange as a successful emerging therapy-case report[J]. Transl Lung Cancer Res, 2021, 10(2): 1039-1046.
[39] Bermas BL, Zaha VG. Mending Broken Hearts: A New Treatment Paradigm for Immune Checkpoint Inhibitor-Induced Myocarditis[J]. Circulation, 2021, 143(8): 767-769.
[40] Balanescu DV, Donisan T, Palaskas N, et al. Immunomodulatory treatment of immune checkpoint inhibitor-induced myocarditis: Pathway toward precision-based therapy[J]. Cardiovasc Pathol, 2020, 47: 107211.
[41] Janbandhu V, Tallapragada V, Patrick R, et al. Hif-1a suppresses ROS-induced proliferation of cardiac fibroblasts following myocardial infarction[J]. Cell Stem Cell, 2022, 29(2): 281-297.e12.
[42] Hua X, Hu G, Hu Q, et al. Single-Cell RNA Sequencing to Dissect the Immunological Network of Autoimmune Myocarditis[J]. Circulation, 2020, 142(4): 384-400.
-

计量
- 文章访问数: 340
- 施引文献: 0



 下载:
下载: