-
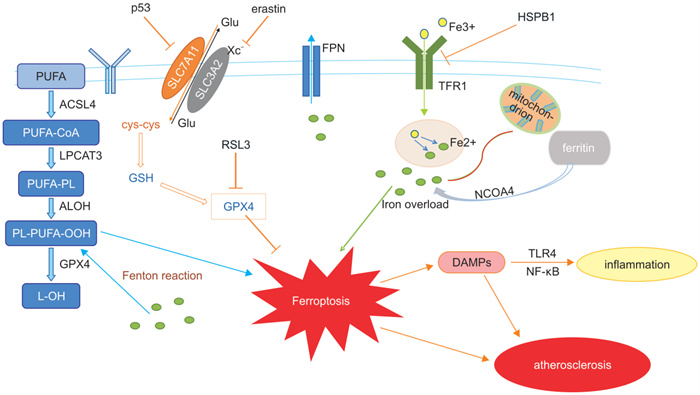
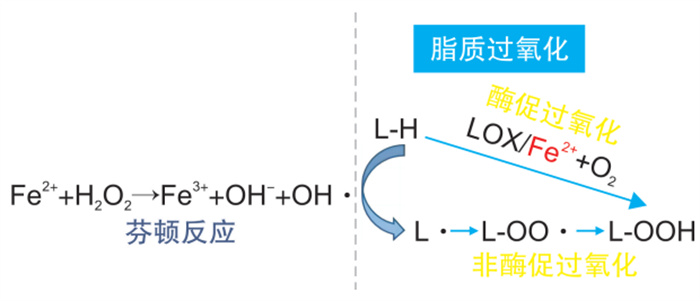
摘要: 动脉粥样硬化是一类以脂质过氧化沉积导致血管内皮损伤为主要特征的血管慢性炎症性疾病,并且也是引起冠心病、心力衰竭和脑卒中等心脑血管疾病的重要因素。铁死亡是以铁依赖性脂质过氧化物增加为主要特点的一种新型细胞死亡形式,业已证明可以通过不同的信号途径参与动脉粥样硬化的发生和发展。本文结合前期的资料与研究进一步探讨铁死亡的机制及其相关通路在动脉粥样硬化中发挥的作用,以为临床上防治动脉粥样硬化提供更多的科学依据。Abstract: Atherosclerosis is a chronic vascular inflammatory disease mainly characterized by lipid peroxidation deposition causing vascular endothelial damage, and is an important factor in coronary heart disease, heart failure, stroke and other cardiovascular and cerebrovascular diseases. Ferroptosis is a new form of cell death characterized by the increase of iron-dependent lipid peroxides, which has been shown to be involved in the occurrence and development of AS through different signaling pathways. In this paper, the mechanism of ferroptosis and its role in atherosclerosis were further discussed.
-
Key words:
- atherosclerosis /
- ferroptosis /
- lipid peroxides /
- cell death
-

-
[1] Wang Y, Zhao Y, Ye T, et al. Ferroptosis signaling and regulators in atherosclerosis[J]. Front Cell Dev Biol, 2021, 9: 809457. doi: 10.3389/fcell.2021.809457
[2] Bai T, Li M, Liu Y, et al. Inhibition of ferroptosis alleviates atherosclerosis through attenuating lipid peroxidation and endothelial dysfunction in mouse aortic endothelial cell[J]. Free Radic Biol Med, 2020, 160: 92-102. doi: 10.1016/j.freeradbiomed.2020.07.026
[3] Lin L, Zhang MX, Zhang L, et al. Autophagy, pyroptosis, and ferroptosis: new regulatory mechanisms for atherosclerosis[J]. Front Cell Dev Biol, 2021, 9: 809955.
[4] Riegman M, Sagie L, Galed C, et al. Ferroptosis occurs through an osmotic mechanism and propagates independently of cell rupture[J]. Nat Cell Biol, 2020, 22(9): 1042-1048. doi: 10.1038/s41556-020-0565-1
[5] Kajarabille N, Latunde-Dada GO. Programmed cell-death by ferroptosis: antioxidants as mitigators[J]. Int J Mol Sci, 2019, 20(19): 110.
[6] Yuan H, Pratte J, Giardina C. Ferroptosis and its potential as a therapeutic target[J]. Biochem Pharmacol, 2021, 186: 114486. doi: 10.1016/j.bcp.2021.114486
[7] Guo Y, Zhang W, Zhou X, et al. Roles of ferroptosis in cardiovascular diseases[J]. Front Cardiovasc Med, 2022, 9: 911564. doi: 10.3389/fcvm.2022.911564
[8] Zhang Y, Xin L, Xiang M, et al. The molecular mechanisms of ferroptosis and its role in cardiovascular disease[J]. Biomed Pharmacother, 2022, 145: 112423. doi: 10.1016/j.biopha.2021.112423
[9] Ilari S, Giancotti LA, Lauro F, et al. Natural antioxidant control of neuropathic pain—exploring the role of mitochondrial SIRT3 Pathway[J]. Antioxidants, 2020, 9(11): 1103. doi: 10.3390/antiox9111103
[10] Oh BM, Lee S, Park GL, et al. Erastin inhibits septic shock and inflammatory gene expression via suppression of the NF-κB pathway[J]. J Clin Med, 2019, 8(12): 2210. doi: 10.3390/jcm8122210
[11] Li J, Xu L, Zuo YX, et al. Potential intervention target of atherosclerosis: Ferroptosis(Review)[J]. Mol Med Rep, 2022, 26(5): 110.
[12] Xie L, Fefelova N, Pamarthi SH, et al. Molecular mechanisms of ferroptosis and relevance to cardiovascular disease[J]. Cells, 2022, 11(17): 2726. doi: 10.3390/cells11172726
[13] Murphy MP. How mitochondria produce reactive oxygen species[J]. Biochem J, 2009, 417(1): 1-13. doi: 10.1042/BJ20081386
[14] Li C, Dong X, Du W, et al. LKB1-AMPK axis negatively regulates ferroptosis by inhibiting fatty acid synthesis[J]. Signal Transduct Target Ther, 2020, 5(1): 187. doi: 10.1038/s41392-020-00297-2
[15] Gao M, Yi J, Zhu J, et al. Role of mitochondria in ferroptosis[J]. Mol Cell, 2019, 73(2): 354-363. e3. doi: 10.1016/j.molcel.2018.10.042
[16] Lee H, Zandkarimi F, Zhang Y, et al. Energy-stress-mediated AMPK activation inhibits ferroptosis[J]. Nat Cell Biol, 2020, 22(2): 225-234. doi: 10.1038/s41556-020-0461-8
[17] Shimada K, Skouta R, Kaplan A, et al. Global survey of cell death mechanisms reveals metabolic regulation of ferroptosis[J]. Nat Chem Biol, 2016, 12(7): 497-503. doi: 10.1038/nchembio.2079
[18] DeHart DN, Fang D, Heslop K, et al. Opening of voltage dependent anion channels promotes reactive oxygen species generation, mitochondrial dysfunction and cell death in cancer cells[J]. Biochem Pharmacol, 2018, 148: 155-162. doi: 10.1016/j.bcp.2017.12.022
[19] Dodson M, Castro-Portuguez R, Zhang DD. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis[J]. Redox Biol, 2019, 23: 101107. doi: 10.1016/j.redox.2019.101107
[20] Sun X, Ou Z, Chen R, et al. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells[J]. Hepatology, 2016, 63(1): 173-184. doi: 10.1002/hep.28251
[21] Chen X, Li J, Kang R, et al. Ferroptosis: machinery and regulation[J]. Autophagy, 2021, 17(9): 2054-2081. doi: 10.1080/15548627.2020.1810918
[22] Shin D, Kim EH, Lee J, et al. Nrf2 inhibition reverses resistance to GPX4 inhibitor-induced ferroptosis in head and neck cancer[J]. Free Radic Biol Med, 2018, 129: 454-462. doi: 10.1016/j.freeradbiomed.2018.10.426
[23] Chen D, Tavana O, Chu B, et al. NRF2 is a major target of ARF in p53-independent tumor suppression[J]. Mol Cell, 2017, 68(1): 224-232. e4. doi: 10.1016/j.molcel.2017.09.009
[24] Chepikova OE, Malin D, Strekalova E, et al. Lysine oxidase exposes a dependency on the thioredoxin antioxidant pathway in triple-negative breast cancer cells[J]. Breast Cancer Res Treat, 2020, 183(3): 549-564. doi: 10.1007/s10549-020-05801-4
[25] Sun X, Ou Z, Chen R, et al. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells[J]. Hepatology, 2016, 63(1): 173-184. doi: 10.1002/hep.28251
[26] Yu W, Liu W, Xie D, et al. High level of uric acid promotes atherosclerosis by targeting nrf2-mediated autophagy dysfunction and ferroptosis[J]. Oxidative Medicine and Cellular Longevity, 2022, 2022: 1-21.
[27] Bersuker K, Hendricks JM, Li Z, et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis[J]. Nature, 2019, 575(7784): 688-692. doi: 10.1038/s41586-019-1705-2
[28] Mishima E, Ito J, Wu Z, et al. A non-canonical vitamin K cycle is a potent ferroptosis suppressor[J]. Nature, 2022, 608(7924): 778-783. doi: 10.1038/s41586-022-05022-3
[29] Bromberg JF, Wrzeszczynska MH, Devgan G, et al. Stat3 as an oncogene. [J]. Cell, 1999, 98(3): 295-303. doi: 10.1016/S0092-8674(00)81959-5
[30] Mohan CD, Bharathkumar H, Bulusu KC, et al. Development of a novel azaspirane that targets the Janus kinase-signal transducer and activator of transcription(STAT)pathway in hepatocellular carcinoma in vitro and in vivo[J]. J Biol Chem, 2014, 289(49): 34296-34307. doi: 10.1074/jbc.M114.601104
[31] Santoni M, Massari F, Del Re M, et al. Investigational therapies targeting signal transducer and activator of transcription 3 for the treatment of cancer[J]. Expert Opin Investig Drugs, 2015, 24(6): 809-824. doi: 10.1517/13543784.2015.1020370
[32] Knight RA, Scarabelli TM, Stephanou A. STAT transcription in the ischemic heart[J]. JAK-STAT, 2014, 1(2): 111-117.
[33] Siveen KS, Sikka S, Surana R, et al. Targeting the STAT3 signaling pathway in cancer: role of synthetic and natural inhibitors[J]. Biochim Biophys Acta, 2014, 1845(2): 136-154.
[34] Wang X, Chen L, Liu J, et al. In vivo treatment of rat arterial adventitia with interleukin-1β induces intimal proliferation via the JAK2/STAT3 signaling pathway[J]. Mol Med Rep, 2016, 13(4): 3451-3458. doi: 10.3892/mmr.2016.4982
[35] Fu X, Sun Z, Long Q, et al. Glycosides from buyang huanwu decoction inhibit atherosclerotic inflammation via JAK/STAT signaling pathway[J]. Phytomedicine, 2022, 105: 154385. doi: 10.1016/j.phymed.2022.154385
[36] Ouyang S, You J, Zhi C, et al. Ferroptosis: the potential value target in atherosclerosis[J]. Cell Death Dis, 2021, 12(8): 782. doi: 10.1038/s41419-021-04054-3
[37] Ma WQ, Sun XJ, Zhu Y, et al. Metformin attenuates hyperlipidaemia-associated vascular calcification through anti-ferroptotic effects[J]. Free Radic Biol Med, 2021, 165: 229-242. doi: 10.1016/j.freeradbiomed.2021.01.033
[38] Iwabayashi M, Taniyama Y, Sanada F, et al. Inhibition of Lp(a)-induced functional impairment of endothelial cells and endothelial progenitor cells by hepatocyte growth factor[J]. Biochem Biophys Res Commun, 2012, 423(1): 79-84. doi: 10.1016/j.bbrc.2012.05.086
[39] Yin H, Xu L, Porter NA. Free radical lipid peroxidation: mechanisms and analysis[J]. Chem Rev, 2011, 111(10): 5944-5972. doi: 10.1021/cr200084z
[40] Fang X, Wang H, Han D, et al. Ferroptosis as a target for protection against cardiomyopathy[J]. Proc Natl Acad Sci U S A, 2019, 116(7): 2672-2680. doi: 10.1073/pnas.1821022116
[41] He L, Liu YY, Wang K, et al. Tanshinone ⅡA protects human coronary artery endothelial cells from ferroptosis by activating the NRF2 pathway[J]. Biochem Biophys Res Commun, 2021, 575: 1-7. doi: 10.1016/j.bbrc.2021.08.067
[42] Feng Y, Madungwe NB, Imam Aliagan AD, et al. Liproxstatin-1 protects the mouse myocardium against ischemia/reperfusion injury by decreasing VDAC1 levels and restoring GPX4 levels[J]. Biochem Biophys Res Commun, 2019, 520(3): 606-611. doi: 10.1016/j.bbrc.2019.10.006
[43] Li J, Gao Z, Zhang L, et al. Qing-Xin-Jie-Yu Granule for patients with stable coronary artery disease(QUEST Trial): A multicenter, double-blinded, randomized trial[J]. Complement Ther Med, 2019, 47: 102209. doi: 10.1016/j.ctim.2019.102209
[44] Zhang J, Wang X, Guan B, et al. Qing-Xin-Jie-Yu Granule inhibits ferroptosis and stabilizes atherosclerotic plaques by regulating the GPX4/xCT signaling pathway[J]. J Ethnopharmacol, 2023, 301: 115852. doi: 10.1016/j.jep.2022.115852
[45] 潘红波, 李明杰, 满雨楠, 等. 血管紧张素转换酶2在动脉粥样硬化中的研究进展[J]. 临床心血管病杂志, 2023, 39(12): 972-976. https://lcxxg.whuhzzs.com/article/doi/10.13201/j.issn.1001-1439.2023.12.013
[46] Wang X, Yan K, Wen C, et al. Simvastatin combined with resistance training improves outcomes in patients with chronic heart failure by modulating mitochondrial membrane potential and the janus kinase/signal transducer and activator of transcription 3 signaling pathways[J]. Cardiovascular Therapeutics, 2022, 2022: 1-9.
[47] Wu X, Pan J, Yu JJ, et al. DiDang decoction improves mitochondrial function and lipid metabolism via the HIF-1 signaling pathway to treat atherosclerosis and hyperlipidemia[J]. J Ethnopharmacol, 2023, 308: 116289. doi: 10.1016/j.jep.2023.116289
-

计量
- 文章访问数: 398
- 施引文献: 0



 下载:
下载: