Effects of semaglutide on cardiovascular outcomes in patients with obesity with or without diabetes: a meta-analysis based on randomized controlled trials
-
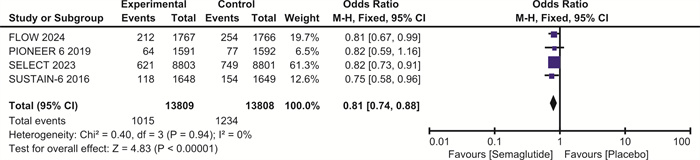
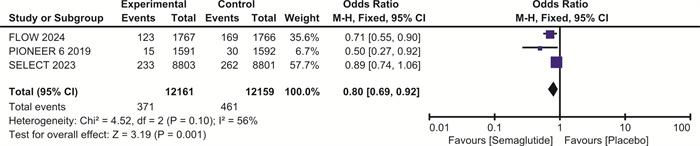
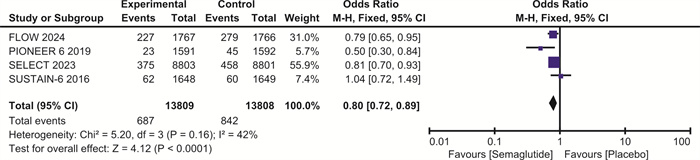
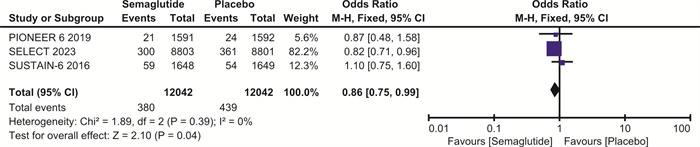
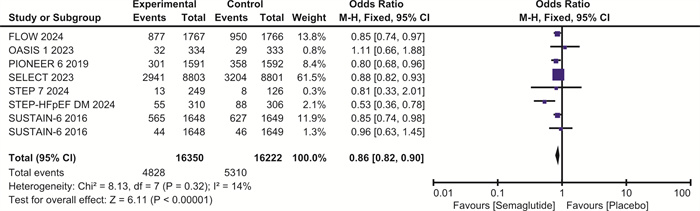
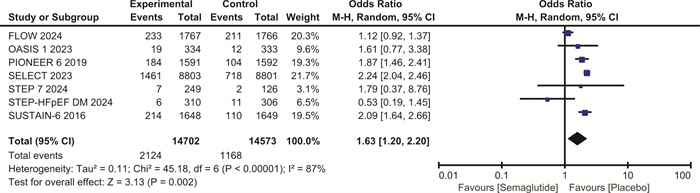
摘要: 目的 探讨司美格鲁肽对伴或不伴糖尿病的肥胖症患者心血管预后的影响。方法 检索Pubmed、Embase和Cochrane图书馆的数据库,纳入体重指数(BMI)>30 kg/m2、伴或不伴糖尿病的患者,使用RevMan 5.4进行荟萃分析。结果 最终纳入7项随机对照研究,共计29 275例。与安慰剂相比,在伴或不伴糖尿病的肥胖症患者中,司美格鲁肽显著减少主要不良心血管事件(major adverse cardiovascular events,MACE)(OR=0.81,95%CI:0.74~0.88,P < 0.001)、全因死亡(OR=0.80,95%CI:0.72~0.89,P < 0.001)、心血管死亡(OR=0.80,95%CI:0.69~0.92,P=0.001)、心力衰竭(OR=0.86,95%CI:0.75~0.99,P=0.04)和严重不良事件(OR=0.86,95%CI:0.82~0.90,P < 0.001)。与安慰剂相比,司美格鲁肽导致治疗中断的不良事件发生率明显升高(OR=1.63,95%CI:1.20~2.20,P=0.002)。结论 司美格鲁肽可降低MACE、全因死亡、心血管死亡、心力衰竭以及严重不良事件的发生率,但会增加治疗中断的不良事件。Abstract: Objective To explore the effect of semaglutide on cardiovascular outcomes in obese patients with or without diabetes.Methods Databases from Pubmed, Embase and the Cochrane Library were searched. We recruited patients with a body mass index(BMI) greater than 30 kg/m2, with or without diabetes. A meta-analysis was performed using RevMan 5.4.Results A total of 7 randomized controlled trials involving 29 275 participants were included. Compared to placebo, semaglutide significantly reduced major adverse cardiovascular events(MACE) in obese patients with or without diabetes(OR=0.81, 95%CI: 0.74 to 0.88, P < 0.001). It also reduced all-cause mortality(OR=0.80, 95%CI: 0.72 to 0.89, P < 0.001), cardiovascular mortality(OR=0.80, 95%CI: 0.69 to 0.92, P=0.002), heart failure(OR=0.86, 95%CI: 0.75 to 0.99, P=0.04), and serious adverse events(OR=0.86, 95%CI: 0.82 to 0.90, P < 0.001). However, semaglutide was associated with a significantly higher incidence of adverse events leading to treatment discontinuation compared to placebo(OR=1.63, 95%CI: 1.20 to 2.20, P=0.002).Conclusion Semaglutide may reduce the incidence of MACE, all-cause mortality, cardiovascular mortality, heart failure, and serious adverse events, but it increases the risk of adverse events leading to treatment discontinuation.
-
Key words:
- semaglutide /
- cardiovascular outcomes /
- obesity /
- diabetes
-

-
表 1 纳入研究的基本特征
Table 1. Basic information of included studies
例(%), X±S 项目 SUSTAIN-6 (2016)[17] PIONEER 6 (2019)[18] SELECT (2023)[19] OASIS 1 (2023)[20] STEP-HFpEF DM (2024)[21] FLOW (2024)[22] STEP 7 (2024)[23] 用药方式 每周1次(0.5或1.0 mg)或安慰剂,疗程104周 每天1次口服(目标剂量14 mg)或安慰剂 每周1次,每次0.24 mg,每4周增加1次,直至16周后达到2.4 mg的目标剂量 口服,剂量增至50 mg,或相同剂量的安慰剂,每天1次,连续68周,同时进行生活方式干预 前4周,每周1次,每次0.25 mg,后每4周增加1次,直至16周后达到2.4 mg的目标剂量 采用为期8周的剂量递增方案,剂量递增(只要不出现不可接受的不良反应)从每周0.25 mg开始,持续4周,再持续4周,每周0.5 mg,然后在剩余的治疗期内维持每周1.0 mg 每周1次皮下注射2.4 mg或安慰剂,持续44周,同时接受饮食和体育锻炼干预 例数 3 297 3 183 17 604 667 616 3 533 375 男性 2 002(60.7) 2 177(68.4) 12 728(72.3) 182(27) 343(55.7) 2 464(69.7) 205(55) 年龄/岁 64.6±7.4 66±7 61.6±8.9 50±13 69±6 66.6±9.0 41±11 体重/kg 92.1±20.6 90.9±21.2 96.6±17.7 105.4±22.2 89.6±20.5 96.4±17.7 BMI/(kg/m2) 32.3±6.5 33.3±5.0 37.5±6.5 36.9±4.6 32.0±6.3 34±4.8 2型糖尿病 100 100 0 0 100 100 糖化血红蛋白/% 8.7±1.4 8.2±1.6 5.8±0.3 5.6±0.3 6.8±0.9 7.8±1.3 6.3±1.1 收缩压/mmHg 135.6±17.1 135±18 131.0±15.4 129±15 138.6±15.8 127±14 舒张压/mmHg 77.1±9.8 76±10 79.3±9.9 82±11 76.4±10.0 84±10 低密度脂蛋白/(mg/dL) 82.4±45.5 78.0±43.1 78.0±42.8 111.5±32.7 100±33 既往心肌梗死 1 072(32.5) 11 900(67.6) 既往卒中 491(14.9) 3 134(17.8) 808(22.9) 既往心力衰竭 777(23.6) 616(100) 678(19.2) 随访时间/年 2 1.6 4 1.3 1 3.4 0.8 -
[1] Mahmood SS, Levy D, Vasan RS, et al. The Framingham Heart Study and the epidemiology of cardiovascular disease: a historical perspective[J]. Lancet, 2014, 383(9921): 999-1008. doi: 10.1016/S0140-6736(13)61752-3
[2] Leong DP, Joseph PG, McKee M, et al. Reducing the Global Burden of Cardiovascular Disease, Part 2: Prevention and Treatment of Cardiovascular Disease[J]. Circ Res, 2017, 121(6): 695-710. doi: 10.1161/CIRCRESAHA.117.311849
[3] Clinical Guidelines on the Identification. Evaluation, and treatment of overweight and obesity in adults—the evidence report[J]. Nat Inst Health Obes Res, 1998, 6(Suppl 2): 51S-209S.
[4] Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States[R]. Atlanta, 2014.
[5] Bhupathiraju SN, Hu FB. Epidemiology of Obesity and Diabetes and Their Cardiovascular Complications[J]. Circ Res, 2016, 118(11): 1723-1735. doi: 10.1161/CIRCRESAHA.115.306825
[6] Piché ME, Tchernof A, Després JP. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases[J]. Circ Res, 2020, 126(11): 1477-1500. doi: 10.1161/CIRCRESAHA.120.316101
[7] 赵灿, 王刚, 刘霄燕, 等. BMI对慢性心力衰竭的预后价值[J]. 临床心血管病杂志, 2024, 40(3): 194-198. doi: 10.13201/j.issn.1001-1439.2024.03.007
[8] Hall S, Isaacs D, Clements JN. Pharmacokinetics and Clinical Implications of Semaglutide: A New Glucagon-Like Peptide(GLP)-1 Receptor Agonist[J]. Clin Pharmacokinet, 2018, 57(12): 1529-1538. doi: 10.1007/s40262-018-0668-z
[9] Overgaard RV, Hertz CL, Ingwersen SH, et al. Levels of circulating semaglutide determine reductions in HbA1c and body weight in people with type 2 diabetes[J]. Cell Rep Med, 2021, 2(9): 100387. doi: 10.1016/j.xcrm.2021.100387
[10] Ahmann AJ, Capehorn M, Charpentier G, et al. Efficacy and safety of once-weekly semaglutide versus exenatide ER in subjects with type 2 diabetes(SUSTAIN 3): a 56-week, open-label, randomized clinical trial[J]. Diabetes Care, 2018, 41(2): 258-266. doi: 10.2337/dc17-0417
[11] Aroda VR, Bain SC, Cariou B, et al. Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin(with or without sulfonylureas)in insulin-naive patients with type 2 diabetes(SUSTAIN 4): a randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial[J]. Lancet Diabetes Endocrinol, 2017, 5(5): 355-366. doi: 10.1016/S2213-8587(17)30085-2
[12] Kommu S, Berg RL. Efficacy and safety of once-weekly subcutaneous semaglutide on weight loss in patients with overweight or obesity without diabetes mellitus—A systematic review and meta-analysis of randomized controlled trials[J]. Obes Rev, 2024, 25(9): e13792. doi: 10.1111/obr.13792
[13] Gao X, Hua X, Wang X, et al. Efficacy and safety of semaglutide on weight loss in obese or overweight patients without diabetes: a systematic review and meta-analysis of randomized controlled trials[J]. Front Pharmacol, 2022, 13: 935823. doi: 10.3389/fphar.2022.935823
[14] Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials[J]. BMJ, 2019, 366: l4898.
[15] Lo CK, Mertz D, Loeb M, et al. Newcastle-Ottawa Scale: comparing reviewers' to authors' assessments[J]. BMC Med Res Methodol, 2014, 14: 45. doi: 10.1186/1471-2288-14-45
[16] DerSimonian R, Laird N. Meta-analysis in clinical trials[J]. Control Clin Trials, 1986, 7(3): 177-188. doi: 10.1016/0197-2456(86)90046-2
[17] Marso SP, Bain SC, Consoli A, et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes[J]. N Engl J Med, 2016, 375(19): 1834-1844. doi: 10.1056/NEJMoa1607141
[18] Husain M, Birkenfeld AL, Donsmark M, et al. Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes[J]. N Engl J Med, 2019, 381(9): 841-851. doi: 10.1056/NEJMoa1901118
[19] Lincoff AM, Brown-Frandsen K, Colhoun HM, et al. Semaglutide and Cardiovascular Outcomes in Obesity without Diabetes[J]. N Engl J Med, 2023, 389(24): 2221-2232. doi: 10.1056/NEJMoa2307563
[20] Perkovic V, Tuttle KR, Rossing P, et al. Effects of Semaglutide on Chronic Kidney Disease in Patients with Type 2 Diabetes[J]. N Engl J Med, 2024, 391(2): 109-121. doi: 10.1056/NEJMoa2403347
[21] Kosiborod MN, Petrie MC, Borlaug BA, et al. Semaglutide in Patients with Obesity-Related Heart Failure and Type 2 Diabetes[J]. N Engl J Med, 2024, 390(15): 1394-1407. doi: 10.1056/NEJMoa2313917
[22] Mu Y, Bao X, Eliaschewitz FG, et al. Efficacy and safety of once weekly semaglutide 2.4 mg for weight management in a predominantly east Asian population with overweight or obesity(STEP 7): a double-blind, multicentre, randomised controlled trial[J]. Lancet Diabetes Endocrinol, 2024, 12(3): 184-195. doi: 10.1016/S2213-8587(23)00388-1
[23] Knop FK, Aroda VR, do Vale RD, et al. Oral semaglutide 50 mg taken once per day in adults with overweight or obesity(OASIS 1): a randomised, doubleblind, placebo-controlled, phase 3 trial[J]. Lancet, 2023, 402(10403): 705-719. doi: 10.1016/S0140-6736(23)01185-6
[24] Husain M, Consoli A, Remigis AD, et al. Semaglutide reduces cardiovascular events regardless of metformin use: a post hoc subgroup analysis of SUSTAIN 6 and PIONEER 6[J]. Cardiovasc Diabetol, 2022, 21(1): 64. doi: 10.1186/s12933-022-01489-6
[25] Kosiborod MN, Abildstrøm SZ, Borlaug BA, et al. Semaglutide in Patients with Heart Failure with Preserved Ejection Fraction and Obesity[J]. N Engl J Med, 2023, 389(12): 1069-1084. doi: 10.1056/NEJMoa2306963
[26] Butler J, Abildstrøm SZ, Borlaug BA, et al. Semaglutide in Patients With Obesity and Heart Failure Across Mildly Reduced or Preserved Ejection Fraction[J]. J Am Coll Cardiol, 2023, 82(22): 2087-2096. doi: 10.1016/j.jacc.2023.09.811
[27] Andreadis P, Karagiannis T, Malandris K, et al. Semaglutide for type 2 diabetes mellitus: a systematic review and meta-analysis[J]. Diabetes Metab Syndr, 2022, 16(6): 102511. doi: 10.1016/j.dsx.2022.102511
[28] Arastu N, Cummins O, Uribe W, et al. Efcacy of subcutaneous semaglutide compared to placebo for weight loss in obese, non-diabetic adults: a systematic review & meta-analysis[J]. Int J Clin Pharm, 2022, 44(4): 852-859. doi: 10.1007/s11096-022-01428-1
[29] Sandhu H, Xu W, Olivieri AV, et al. Once-Weekly Subcutaneous Semaglutide 2.4 mg Injection is Cost-Effective for Weight Management in the United Kingdom[J]. Adv Ther, 2023, 40(3): 1282-1291. doi: 10.1007/s12325-022-02423-8
[30] Evans M, Chubb B, Malkin Samuel JP, et al. Once-weekly semaglutide versus insulin aspart for the treatment of type 2 diabetes in the UK: A long-term cost-effectiveness analysis based on sustain 11[J]. Diabetes Obes Metab, 2023, 25(2): 491-500. doi: 10.1111/dom.14892
[31] Wilding JPH, Batterham RL, Calanna S, et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity[J]. N Engl J Med, 2021, 384(11): 989-1002. doi: 10.1056/NEJMoa2032183
[32] Atef MM, Hafez YM, El-Deeb OS, et al. The cardioprotective effect of human glucagon-like peptide-1 receptor agonist(semaglutide)on cisplatin-induced cardiotoxicity in rats: Targeting mitochondrial functions, dynamics, biogenesis, and redox status pathways[J]. Cell Biochem Funct, 2023, 41(4): 450-460. doi: 10.1002/cbf.3795
[33] Ussher JR, Drucker DJ. Glucagon-like peptide 1 receptor agonists: cardiovascular benefits and mechanisms of action[J]. Nat Rev Cardiol, 2023, 20(7): 463-474. doi: 10.1038/s41569-023-00849-3
[34] Ma X, Liu Z, Ilyas I, et al. GLP-1 receptor agonists(GLP-1RAs): cardiovascular actions and therapeutic potential[J]. Int J Biol Sci, 2021, 17(8): 2050-2068. doi: 10.7150/ijbs.59965
[35] David GV, David S L, Gemma RC, et al. Semaglutide modulates prothrombotic and atherosclerotic mechanisms, associated with epicardial fat, neutrophils and endothelial cells network[J]. Cardiovasc Diabetol, 2024, 23(1): 1. doi: 10.1186/s12933-023-02096-9
[36] Li Q, Tuo X, Li B, et al. Semaglutide attenuates excessive exercise-induced myocardial injury through inhibiting oxidative stress and inflammation in rats[J]. Life Sci, 2020, 250: 117531. doi: 10.1016/j.lfs.2020.117531
[37] Reis-Barbosa PH, Marcondes-de-Castro IA, Marinho TS. The mTORC1/AMPK pathway plays a role in the beneficial effects of semaglutide(GLP-1 receptor agonist)on the liver of obese mice[J]. Clin Res Hepatol Gastroenterol, 2022, 46(6): 101922. doi: 10.1016/j.clinre.2022.101922
[38] Zhu Q, Luo Y, Wen Y, et al. Semaglutide inhibits ischemia/reperfusion-induced cardiomyocyte apoptosis through activating PKG/PKCε/ERK1/2 pathway[J]. Biochem Biophys Res Commun, 2023, 647: 1-8. doi: 10.1016/j.bbrc.2023.01.049
[39] Sattar N, Lee MMY, Kristensen SL, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials[J]. Lancet Diabetes Endocrinol, 2021, 9(10): 653-662. doi: 10.1016/S2213-8587(21)00203-5
[40] Bethel MA, Patel RA, Merrill P, et al. Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a meta-analysis[J]. Lancet Diabetes Endocrinol, 2018, 6(2): 105-113. doi: 10.1016/S2213-8587(17)30412-6
[41] Noda K, Kato T, Nomura N, et al. Semaglutide is efective in type 2 diabetes and obesity with schizophrenia[J]. Diabetol Int, 2022, 13(4): 693-697. doi: 10.1007/s13340-022-00590-1
[42] Sadek MA, Kandil EA, Sayed NS, et al. Semaglutide, a novel glucagon-like peptide-1 agonist, amends experimental autoimmune encephalomyelitis-induced multiple sclerosis in mice: Involvement of the PI3K/Akt/GSK-3β pathway[J]. Int Immunopharmacol, 2023, 115: 109647. doi: 10.1016/j.intimp.2022.109647
[43] Manuel RG, Matthew JA, Jesús FS, et al. Improved health-related quality of life with semaglutide in people with non-alcoholic steatohepatitis: A randomised trial[J]. Aliment Pharmacol Ther, 2023, 58(4): 395-403. doi: 10.1111/apt.17598
-

计量
- 文章访问数: 329
- 施引文献: 0




 下载:
下载: